Abstract
Introduction
The role of postoperative radiotherapy in treating squamous cell carcinoma of the vulva remains controversial. This study evaluated the effect of radiotherapy on the survival of patients with postoperative squamous cell carcinoma of the vulva.
Methods
Clinical and prognostic information on patients diagnosed with vulvar squamous cell carcinoma from 2010 to 2015 was collected from the Surveillance, Epidemiology, and Prognosis (SEER) database. A propensity score matching (PSM) approach was used to balance the differences in clinicopathological characteristics between groups. The impact of postoperative radiotherapy on overall survival (OS) and disease-specific survival (DSS) was assessed.
Results
The study included 3571 patients with squamous cell carcinoma of the vulva, of whom 732 (21.1%) received postoperative radiotherapy. After propensity score matching, multivariate analysis showed that age, race, N stage, and tumor size were independent influences on overall survival and disease-specific survival of patients. Postoperative radiotherapy did not improve patients’ overall survival or disease-specific survival. Further subgroup survival analysis showed that in patients with AJCC stage III, N1 stage, lymph node metastasis, and large tumor diameter (> 3.5 cm), postoperative radiotherapy resulted in a significant improvement in overall patient survival.
Conclusion
Postoperative radiotherapy is not indicated for all patients with postoperative vulvar cancer and has improved survival outcomes only for patients with AJCC stage III, N1, lymph node metastases and large tumor diameter (> 3.5 cm).
Similar content being viewed by others
Introduction
Vulvar cancer is a rare gynecologic malignancy, accounting for 5% of gynecologic malignancies, and it is most common in older women [1]. Over the past decade, the overall incidence of vulvar cancer has increased by an average of 4.6% every five years [2]. According to reports, there were 6120 new vulvar cancer cases in the United States in 2020 and 1350 deaths [3]. The most common pathological type of vulvar cancer is vulvar squamous cell carcinoma, followed by melanoma. Vulvar bleeding, pain, and sexual dysfunction are the main clinical symptoms of the disease, which seriously affect the quality of life and physical and mental health of patients.
Surgery is the primary method of treatment for vulvar cancer. Compared to radical vulvectomy, partial radical vulvectomy has become the procedure of choice in recent years due to fewer postoperative complications [4,5,6]. The main reasons currently affecting the prognosis of patients with vulvar cancer are postoperative complications and a high rate of local recurrence. Although postoperative radiotherapy is thought to enhance the control of postoperative tumors, the role of postoperative radiotherapy is not fully understood due to the low incidence of vulvar cancer and limited clinical studies in large samples.
This study explored the impact of postoperative radiotherapy on the prognosis of patients with vulvar squamous cell carcinoma using data from the SEER database. To minimize selection bias in the included sample, propensity score matching was performed to balance the distribution of baseline clinicopathological variables between the two cohort populations.
Materials and methods
Patient data for this study were obtained from the National Cancer Institute’s SEER database between 2010 and 2015. The SEER database is a sizeable cancer-related database in the United States [7, 8], containing information on the incidence, treatment, and prognosis of cancer patients collected by multiple institutions since 1973. We used SEER*Stat software (version 8.3.9) to extract eligible cases from the database.
For patients with pathologically confirmed vulvar cancer from 2010 to 2015, the primary vulvar site cases were obtained using the “primary site” variable. The histological subtype of vulvar cancer was determined using the variable “ICD-0-3 Hist/Behav, malignant”. We extracted demographic variables, including age at diagnosis, race, primary site, histological type, tumor grade, tumor size, surgery, radiotherapy, chemotherapy, lymph node status, marital status, cause of death, survival (from diagnosis to end or last follow-up), TNM staging, and AJCC staging (7th edition).
Since the distribution of patients in the SEER database is not random, selection bias of baseline characteristics may affect the final results. To reduce the effects of data bias and confounding variables, propensity score matching (PSM) was used to adjust for potential baseline confounders [9]. Scores were calculated based on the nearest neighbor 1:1 matching algorithm, while logistic regression was used to construct the model. The following baseline covariates were considered: patient age, race, pathological grade, tumor grade, tumor size, T-stage, N-stage, M-stage, whether lymph nodes were metastatic, and marital status, with a set caliper of 0.5, and patients who received postoperative radiotherapy were matched to other patients.
Inclusion criteria for this study: (1) patients without distant metastases at diagnosis; (2) positive histopathological confirmation; (3) undergoing surgical treatment. Exclusion criteria: (1) incomplete patient data, such as age at diagnosis, radiotherapy records, survival status and duration; (2) not treated with surgery; (3) non-squamous cell carcinoma pathological types; (4) patients with distant metastases from vulvar cancer.
Based on the above criteria, 3571 cases were finally included in this study (Fig. 1 and Additional File Fig S1). Based on the definition provided by SEER, distant metastases are defined as metastases of vulvar cancer lesions to the bladder or rectum, the proximal 2/3 of the urethra, the pelvis, and/or distant lymph nodes. Because SEER provides limited information on specific treatment, surgery includes all types of surgery and radiation therapy includes all types of radiation therapy.
Comparison of overall survival OS (A) and disease-specific survival DSS (B) between patients in the post-operative radiation therapy and no post-operative radiation therapy groups before PSM;Comparison of overall survival OS (C) and disease-specific survival DSS (D) between patients in the post-operative radiation therapy (PORT) and no post-operative radiation therapy groups after PSM.
The tumors were classified into four different size groups by ranking the lesion sizes of the cases included in this study, using the interquartile spacing as the cut-off value, in which > 75% of the tumors (> 3.5 cm in diameter) were defined as large tumors, which is closer to the locally advanced tumors (> 4 cm in diameter) defined in the 2022 NCCN vulvar cancer guidelines, and also more in line with the actual situation of this study.
The primary and secondary endpoints were overall survival (OS) and disease-specific survival (DSS). Categorical variables were analyzed using the chi-square test. The Kaplan-Meier method was used to produce survival curves and the log-rank test to analyze the differences between the survival outcomes of the two curves. Univariate and multivariate Cox scale risk models were used to identify risk factors affecting OS and DSS. A Cox proportional risk model was used in the subgroup analysis to determine the population of patients who might benefit from radiotherapy. It is considered statistically significant when the bilateral p-value is less than 0.05. The above statistical analysis was calculated using SPSS software (version 24.0) and R software (version 4.0.2).
Results
This study included 3571 patients who underwent surgery and were diagnosed with vulvar squamous cell carcinoma between 2010 and 2015, of whom 732 (20.4%) received radiotherapy. Table 1 shows the demographic and clinicopathological characteristics between the group receiving postoperative radiotherapy and the group not receiving postoperative radiotherapy. Prior to propensity score matching (PSM), significant differences existed between the two groups in terms of race, tumor cell grading, disease stage, tumor size, chemotherapy status, lymph node metastasis, and lymph node surgical distribution. After balancing the baseline characteristics of the two PSM groups, there were 385 patients in each group. All characteristics were balanced between the cohorts using matched propensity scores (Table 1).
Before PSM, the 5-year OS and DSS were significantly higher in the no-radiotherapy group (OS: 68.5%; DSS: 88.5%) than in the radiotherapy group (OS: 48.6%; DSS: 62.0%, both P < 0. 001); there were multiple significant crossover points in the K-M survival analysis curves between the radiotherapy and no-radiotherapy groups after PSM, suggesting that the model was a non-equivalent regression model, and by log - rank test, there was no significant difference in OS and DSS between the radiotherapy and no-radiation groups (P = 0.81, P = 0.56) (Fig. 1). Multivariate analysis showed (Tables 2 and 3) that postoperative radiotherapy had no significant effect on OS (HR 1.138, 95% CI 0.940–1.377, P value 0.186) and DSS (HR 1.006, 95% CI 0.797–1.270, P value 0.959) of patients. Age (HR 2.027, 95% CI 1.698–2.420, p < 0.001; HR 1.937, 95% CI 1.555–2.412,p < 0.001), race (HR 0.792, 95% CI 0.666–0.942, p = 0.008; HR 0.713, 95% CI 0.564–0.901, p = 0.005), AJCC staging (HR 1.199, 95% CI 1.014–1.419, p = 0.034; HR 1.445, 95% CI 1. 191-1.752, p < 0.001), N staging (HR 1.417, 95% CI 1.140–1.760, p = 0.002; HR 1.579 95% CI 1. 191-1.937, p = 0.001) and tumor size (HR 1.240, 95% CI 1.113–1.381, p < 0.001; HR 1.192, 95% CI 1.041–1.365, p = 0. 011) were independent factors affecting patients’ OS and DSS.
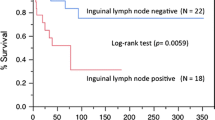
Subgroup analysis showed a significant benefit of postoperative radiotherapy in improving OS in patients with AJCC grade III and N1 (P = 0.048, P = 0.004). For patients with lymph node metastasis, postoperative radiotherapy significantly improved OS (P = 0.035). For patients with large tumor size (> 3.5 cm in diameter), postoperative radiotherapy had better OS (P = 0.021) (Figs. 2 and 3).
Summary of survival curves for subgroup analysis. A: Comparison of survival outcomes in patients aged less than 49 years who received postoperative radiotherapy with or without; B: Comparison of survival outcomes in AJCC stage I patients treated with and without postoperative radiotherapy; C: Comparison of survival outcomes in AJCC stage III patients treated with and without postoperative radiotherapy; D: Comparison of survival outcomes of AJCC stage N0 patients treated with or without postoperative radiotherapy; E: Comparison of survival outcomes in AJCC stage N1 patients treated with or without postoperative radiotherapy; F: Comparison of survival outcomes in patients with lymph node metastases treated with and without postoperative radiotherapy; G: Comparison of survival outcomes in patients with non-metastatic lymph nodes treated with and without postoperative radiotherapy; H: Comparison of survival outcomes in patients with small tumor lesions treated with and without postoperative radiotherapy; J: Comparison of survival outcomes in patients with large tumor lesions treated with and without postoperative radiotherapy
Discussion
Owing to the low incidence of vulvar squamous cell carcinoma, it is challenging to study the prognostic impact of postoperative radiotherapy on patients with vulvar squamous cell carcinoma using large randomized controlled trials [10]. In recent years, oncologic radiotherapy techniques have developed rapidly [11, 12]. Their role in treating patients with vulvar cancer has received increasing attention [13]; however, the effect of postoperative radiotherapy on the survival of patients with squamous cell carcinoma of the vulva is unclear.
Due to differences in tumor biology, several previous single-center studies have reported the role of radiotherapy in treating patients with vulvar cancer [14]. Ignatov T et al. recruited 257 patients with vulvar squamous cell carcinoma and divided the enrolled patients into lymph node metastasis positive and negative groups and found that adjuvant radiotherapy did not improve the prognosis of lymph node negative patients [15]. Meanwhile, Macit Arvas et al. studied 107 patients with postoperative vulvar cancer with long-term follow-up. They found that postoperative radiotherapy only improved the 5-year overall survival of patients with positive surgical margins [16].
To obtain data closely related to current clinical practice, clinical information on patients with vulvar cancer from the SEER database from 2010 to 2015 was collected for this study, and PSM was used to balance the clinical characteristics of the two samples, which could more closely resemble a randomized controlled study and make the results more reliable. According to the inclusion criteria, 3571 patients with squamous cell carcinoma of the vulva were included in this study. The effect of postoperative radiotherapy on survival could be directly analyzed by 1:1 PSM matching according to whether they received postoperative radiotherapy or not. The results showed that postoperative radiotherapy failed to improve OS and DSS in patients with vulvar squamous carcinoma.
Regarding prognostic factors of vulvar cancer, VULCAN retrospectively analyzed the clinicopathological characteristics and prognosis of 1727 patients with vulvar cancer. Multivariate analysis showed that tumor stage, tumor size and ethnicity profile were independent prognostic factors affecting patients’ OS. In addition, the French Society of Radiation Oncology specified that the main factors affecting the postoperative prognosis of patients with vulvar cancer include lymph node involvement, tumor stage, and patient age [17]. Our cohort underwent multivariate COX regression analysis after PSM analysis, and tumor size was also a factor influencing patient prognosis; however, postoperative radiotherapy in patients with squamous vulvar cancer was not an independent influencing factor.
To further clarify the subgroup population benefiting from postoperative radiotherapy for vulvar cancer and to guide personalized clinical treatment, we conducted a subgroup survival analysis, which showed that among patients receiving radiotherapy, OS was significantly higher in patients with AJCC stage III, N1 stage, lymph node metastasis, and large tumor diameter. A recent study analyzing factors related to overall survival and disease recurrence in patients with vulvar cancer pointed out that the size of the primary lesion is an important reference for overall survival [18]. Tumor diameter is closely related to lymph node metastasis. When the lesion diameter is less than 2 cm, the lymph node metastasis rate is about 23%, while when the lesion diameter is greater than 2 cm, the lymph node metastasis rate is as high as 47% [19]. Adjuvant radiotherapy has been shown to significantly prolong the overall survival of patients with advanced disease [17, 20]. In a retrospective analysis of 54 cases of vulvar cancer by S C Han 1 et al., adjuvant radiotherapy was found to significantly improve disease-specific survival (P = 0.03) and overall survival (P = 0.04) in patients with locally advanced vulvar cancer [21]. In this study, survival analysis of a PSM-matched cohort showed a significant improvement in overall survival after postoperative radiotherapy in patients with advanced (AJCC stage III) vulvar cancer, which may be related to the better control of disease recurrence with adjuvant radiotherapy.
It has been confirmed that lymph node status, including whether the lymph nodes are metastatic and the number of positive lymph nodes, affects the recurrence of vulvar cancer and the prognosis of patients [22, 23]. Meanwhile, the International Federation of Obstetrics and Gynecology guidelines recommend adjuvant radiotherapy as a necessary treatment for lymph node-positive vulvar cancer patients. For patients with lymph node-positive vulvar cancer, adjuvant radiotherapy was associated with a lower risk of local recurrence (25.5% vs. 15.8%) [24]. A recent AGO-CaRE 1 study found that adjuvant radiotherapy significantly improved progression-free survival in lymph node-positive vulvar cancer [10].
Our study has some limitations: first, it is a retrospective study with some selection bias, and PSM minimized the influence of confounding factors on the results. Secondly, the SEER database does not contain indicators of specific radiotherapy methods, radiotherapy sites, and radiotherapy regimens, which also affect patient prognosis.
In conclusion, an analysis of vulvar cancer data from the SEER database showed that postoperative radiotherapy failed to improve overall and disease-specific survival in all patients with vulvar squamous cell carcinoma. Postoperative radiotherapy improved survival outcomes only in patients with AJCC stage III, N1, large tumor diameter, and lymph node metastases.
Data Availability
Data from the SEER program is available for public. The data supporting the conclusions of this article are available in the Surveillance Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/).
References
Tan A, Bieber AK, Stein JA, Pomeranz MK. Diagnosis and management of vulvar cancer: a review. J Am Acad Dermatol. 2019;81(6):1387–96.
Zapardiel I, Iacoponi S, Coronado PJ, Zalewski K, Chen F, Fotopoulou C, Dursun P, Kotsopoulos IC, Jach R, Buda A, et al. Prognostic factors in patients with vulvar cancer: the VULCAN study. Int J Gynecol Cancer. 2020;30(9):1285–91.
Michalski BM, Pfeifer JD, Mutch D, Council ML. Cancer of the Vulva: a review. Dermatologic surgery: official publication for American Society for dermatologic surgery [] 2021, 47(2):174–83.
Dellinger TH, Hakim AA, Lee SJ, Wakabayashi MT, Morgan RJ, Han ES. Surgical Management of Vulvar Cancer. J Natl Compr Cancer Network: JNCCN. 2017;15(1):121–8.
Ghoniem K, Shazly SA, Dinoi G, Zanfagnin V, Glaser GE, Mariani A. Sentinel Lymph Nodes and Precision surgery in Gynecologic Cancer. Clin Obstet Gynecol. 2020;63(1):12–23.
He L, Chen G, Li X, Zheng Y, Wu M, Wang H, Liu X, He W, Liu X, Huang S, et al. Safety and feasibility of single-incision radical vulvectomy: a novel approach for the treatment of vulvar cancer. Ann Transl Med. 2021;9(4):320.
Doll KM, Rademaker A, Sosa JA. Practical guide to Surgical Data sets: Surveillance, Epidemiology, and end results (SEER) database. JAMA Surg. 2018;153(6):588–9.
Zhao W, Wang B, Zhao A, Tian Q, Zhang L, Wang L, Zhao X, Yang J, Dong D. The role of radiotherapy in patients with resected ampullary carcinoma: findings based on the SEER database. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2019;21(11):1535–40.
!!!. INVALID CITATION !!! [9, 10].
Mahner S, Jueckstock J, Hilpert F, Neuser P, Harter P, de Gregorio N, Hasenburg A, Sehouli J, Habermann A, Hillemanns P et al. Adjuvant therapy in lymph node-positive vulvar cancer: the AGO-CaRE-1 study. J Natl Cancer Inst 2015, 107(3).
Milliken S, May J, Sanderson PA, Congiu MA, D’Oria O, Golia D’Augè T, Caruso G, Benedetti Panici VDID, Giannini P. Reducing the radicality of surgery for vulvar cancer: are smaller margins safer? Minerva Obstet Gynecol. 2021;73(2):160–5.
Giannini A, D’Oria O, Chiofalo B, Bruno V, Baiocco E, Mancini E, Mancari R, Vincenzoni C, Cutillo G, Vizza E. The giant steps in surgical downsizing toward a personalized treatment of vulvar cancer. J Obstet Gynaecol Res. 2022;48(3):533–40.
Rao YJ, Chin RI, Hui C, Mutch DG, Powell MA, Schwarz JK, Grigsby PW, Markovina S. Improved survival with definitive chemoradiation compared to definitive radiation alone in squamous cell carcinoma of the vulva: a review of the National Cancer Database. Gynecol Oncol. 2017;146(3):572–9.
Raspagliesi F, Hanozet F, Ditto A, Solima E, Zanaboni F, Vecchione F, Kusamura S. Clinical and pathological prognostic factors in squamous cell carcinoma of the vulva. Gynecol Oncol. 2006;102(2):333–7.
Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A. Adjuvant radiotherapy for vulvar cancer with close or positive surgical margins. J Cancer Res Clin Oncol. 2016;142(2):489–95.
Arvas M, Kahramanoglu I, Bese T, Turan H, Sozen I, Ilvan S, Demirkiran F. The role of pathological Margin Distance and prognostic factors after primary surgery in squamous cell carcinoma of the Vulva. Int J Gynecol Cancer. 2018;28(3):623–31.
Chargari C, Petit A, Escande A, Peignaux K, Lafond C, Peiffert D, Hannoun-Lévi JM, Durdux C, Haie-Méder C. Role of radiotherapy in the management of vulvar cancer: recommendations of the french society for radiation oncology. Cancer radiotherapie: journal de la Societe francaise de radiotherapie oncologique. 2022;26(1–2):286–91.
Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol Oncol. 2017;146(1):3–10.
Viswanathan AN, Pinto AP, Schultz D, Berkowitz R, Crum CP. Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol Oncol. 2013;130(3):545–9.
Miljanović-Špika I, Madunić MD, Topolovec Z, Kujadin Kenjereš D, Vidosavljević D. PROGNOSTIC FACTORS FOR VULVAR CANCER. Acta Clin Croatica. 2021;60(1):25–32.
Han SC, Kim DH, Higgins SA, Carcangiu ML, Kacinski BM. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int J Radiat Oncol Biol Phys. 2000;47(5):1235–44.
Van der Zee AG, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, Maggioni A, Gaarenstroom KN, Baldwin PJ, Van Dorst EB, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin oncology: official J Am Soc Clin Oncol. 2008;26(6):884–9.
Te Grootenhuis NC, van der Zee AG, van Doorn HC, van der Velden J, Vergote I, Zanagnolo V, Baldwin PJ, Gaarenstroom KN, van Dorst EB, Trum JW, et al. Sentinel nodes in vulvar cancer: long-term follow-up of the GROningen INternational Study on Sentinel nodes in vulvar cancer (GROINSS-V) I. Gynecol Oncol. 2016;140(1):8–14.
Woelber L, Prieske K, Eulenburg CZ, Corradini S, Petersen C, Bommert M, Blankenstein T, Hilpert F, de Gregorio N, Iborra S, et al. Adjuvant radiotherapy and local recurrence in vulvar cancer - a subset analysis of the AGO-CaRE-1 study. Gynecol Oncol. 2022;164(1):68–75.
Acknowledgements
The authors acknowledge the Surveillance, Epidemiology and Prognosis (SEER) database for providing data for free.
Funding
This research was supported by Shanxi Province Scientific Foundation (Grant No.202103021224352) and Shanxi Province Scientific and Technological Achievements Transformation Foundation (Grant No.201604D132043).
Author information
Authors and Affiliations
Contributions
Miaomiao Li conducted the statistical analysis and drafted the article. Jing Li is responsible for downloading and organizing the data. Zanhong Wang was responsible for the design and guidance of the whole experiment. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Since the SEER data used in this study were de-identified, the research ethics committee of Shanxi Bethune Hospital (Shanxi Academy of Medical Sciences) was exempted from approval. All methods were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing of interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12905_2023_2522_MOESM1_ESM.docx
Additional File Fig S1: The inclusion criteria flowchart of recruited patients in SEER database
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, M., Li, J. & Wang, Z. Prognostic value of postoperative radiotherapy in patients with vulvar squamous carcinoma: findings based on the SEER database. BMC Women's Health 23, 361 (2023). https://doi.org/10.1186/s12905-023-02522-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02522-w