Abstract
Objective
The aim of this study was adopts meta-analysis in evaluating the correlation between TSH and BMD, as well as osteoporosis in the postmenopausal women with normal thyroid function.
Methods
Six databases were searched for articles concerning correlation between TSH and BMD in postmenopausal women. The retrieval time was set from the date of database establishment to November 30, 2020. Revman5.3 and Stata12.0 software were used for meta-analysis.
Results
A total of 19 articles were incorporated. The Summary Fisher’ Z of the correlation between TSH and BMD was 0.16, 95% CI (0.00, 0.32), and the correlation coefficient of Summary Fisher’ Z conversion was 0.158. Study on the relationship between TSH and osteoporosis based on OR demonstrated that the combined OR was 1.76, 95% CI (1.27, 2.45), P < 0.05. The subgroup analyzing results displayed that the risk of osteoporosis of the subjects from community with low TSH was 1.89, 95% CI (1.43, 2.49). The risk of osteoporosis for subjects with low TSH and from hospitals was 1.36, 95% CI (0.46, 3.99); 1.84 for subjects with low TSH and anti-osteoporosis drugs, 95% CI (1.05, 3.22); and 1.74 for those with low TSH but not taking anti-osteoporosis drugs, 95% CI (1.08, 2.82). The dose-response relationship showed that the risk of osteoporosis tended to decrease when TSH was more than 2.5mIu/L.
Conclusion
The serum TSH is positively related with BMD in postmenopausal women, and high TSH (> 2.5 mIu/L) within the normal range is possibly helpful to decrease the risk of osteoporosis in postmenopausal women.
Similar content being viewed by others
Osteoporosis(OP) is a condition resulting in an increased risk of skeletal fractures due to a reduction in the density of bone tissue [1]. and it is common in postmenopausal women. About 30%of postmenopausal women in Asian countries have OP [2]. It is believed that OP serves as the biggest trigger for osteoporotic fracture, and it can elevate the occurrence of fractures, for every 10% decrease in bone mineral density (BMD), the risk of fracture will increase by 2–3 times [3]. Research findings that more than 15% of postmenopausal women may have a hip fracture, and 50% of postmenopausal women may have a lifelong osteoporosis fracture [4]. Therefore, know about the influencing factors of OP in postmenopausal women are of great significance to the public health.
The occurrence of OP is related to gender, age, hormones, etc. [5]. Among them, hormones are believed to be closely related to OP, and thus gained great attention. In postmenopausal women, the decreased in estrogen levels also leads to changes in other hormones, which can significantly increase the incidence of OP [6]. Previous studies have illustrated that the occurrence of OP is related to thyroid hormone. Abe et al. [7] performed the experiments on the TSHR knockout mouse model and found that the TSH level of the TSHR knockout homozygous mice increased, but the BMD level decreased. Exogenous thyroid hormone supplementation did not reverse bone mineral density loss, suggesting that thyroid stimulating hormone (TSH) may be an independent risk factor for OP.
However, we found that there are a lot of controversies about the relationship between serum TSH and BMD of postmenopausal women, based on the published articles or evidence. Cui Xinjie et al. [8] found that serum TSH and lumbar BMD in postmenopausal women with normal thyroid function was positively correlated. However it was reported that serum TSH in postmenopausal women is negatively correlated with lumbar vertebral BMD by Wang Yi [9]. Besides, Yin Fei et al. [10].did not discover the statistical correlation between the serum TSH and the total hip BMD of postmenopausal. There is still no meta-analysis on the exact relationship between serum TSH levels and OP in postmenopausal women with normal thyroid function. Thus, the present systematic review attempted to identify the Relationship between Normal Range of serum thyroid-stimulating hormone and bone mineral density in the postmenopausal women.
Materials and methods
Articles searching strategy
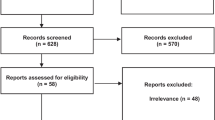
In this study, a total 6 databases were retrieved, we are proficient in English and Chinese, so we searched articles in English in Cochrane Library, PubMed and Web of Science. For Chinese, we searched in CNKI, Wanfang and VIP, and the retrieval time of each database was from database construction to November 30, 2020. See details in Fig. 1.
The key words mainly included the following: postmenopausal women, older women, thyroid-stimulating hormone (TSH), BMD, osteoporosis. Taking Pubmed as an example, the specific retrieval formula is as follows: ((((Postmenopausal women) OR Older women)) AND ((thyroid-stimulating hormone) OR TSH)) AND ((BMD) OR Osteoporosis). When the same article is published in both Chinese and English, we choose the English article for analysis and select according to the inclusion and exclusion criteria.
Inclusion criteria
① Observational studies in both Chinese and English version; ② Research variables are TSH and OP; ③ Outcome index is the correlation relationship and the effective index is r, OR, mean; ④ The research objects in the original articles are postmenopausal women; ⑤ Articles are either in Chinese or English.
Exclusion criteria
① Review articles; ② Spearman correlation coefficient articles; ③ Studies with incomplete data or offer no way to extract the calculated r, OR; ④ Articles unrelated to correlation coefficient between TSH and BMD; ⑤ Abnormal thyroid function.
Statistical analyses
The correlation coefficient of less than 0.5 does not observe the normal distribution. As it moves to more than 0.5, the result remains the same, hence Fisher proposed to use the “Fisher’ Z transformation” formula for conversion, which adapts the correlation coefficient r into a normally distributed variable Z. Since this study adopted meta-analysis based on the Pearson’s correlation coefficient, the “Fisher’ Z transformation” [11] formula could be used for conversion. The specific conversion formula is as follows:
Our study applied Revman5.3 software for statistical analysis, and P < 0.05 was considered statistically significant. EXCEL2010 and Stata12.0 software was used to calculate converted data, draw a dose-response diagram. The above-mentioned formula was adopted to convert the data taking correlation coefficient r as the outcome variable, and thus the Fisher’ Z and standard error (SE) were obtained. Secondly, the Revman5.3 software was applied to perform the inverse variance method, to obtain the summary Fisher’ Z value. Finally, we adopted the formula ③ to gain the combined effect summary r of the correlation coefficient. All of these steps were taken to evaluate the correlation between serum TSH and BMD. In terms of the studies on the relationship between TSH and the risk of OP, most of the articles included only reported the effect size and its 95% CI, and most of them were adjusted for confounding factors. Therefore, the Revman5.3 software can calculate SE and the logarithm of OR (log OR) and then its combined effects observed. Two independent reviewers performed the data extraction, and a third reviewer was consulted for any uncertainties.
Risk of Bias across Studies
Publication Bias
We used a funnel plot and Egger’ s test to evaluate whether there was a publication bias in the included articles.
Sensitivity analysis
The Stata12.0 software was applied for sensitivity analysis. The Chi-square test was performed withα = 0.05 as the significance level; when P < 0.05, the difference was considered statistically significant.
We mainly used the Review Manger 5.3 and Stata12.0 software for data analysis. Heterogeneity is divided into two degrees according to I2, I2 < 50% is low heterogeneity that is acceptable, I2 ≥ 50% is high heterogeneity, andα = 0.05 was applied as the significance level for hypothesis testing of heterogeneity I2. When P < 0.05, I2 ≥ 50%, indicating heterogeneity among multiple studies, the combined effect of OR and its 95% confidence interval is estimated by the random-effects models, and when P > 0.05, I2 < 50%, indicating homogeneity among multiple studies, the fixed-effect model was used to estimate the combined effect and its 95% confidence interval. When the heterogeneity is high, subgroup analysis will be conducted according to the source of the study objects, the detection site of bone mineral density, and the use of anti-osteoporosis drugs in order to find the source of heterogeneity.
The method recommended by the Agency for Health care Research and Quality (AHRQ) is adopted in evaluating the quality of the cross-sectional studies. It contains 11 items, with a maximum score of 11 points. Articles scored 0–3 points, 4–7 points, and 8–11 points are classified into low quality, medium quality, and high-quality respectively (Additional file 3).19 articles were included in this study are of medium quality.
The quality assessment was independently conducted by the first author, and the second author checked and collated the results in detail. Discussed and resolved any disagreement with the third author.
Results
Basic information of the included articles
This study included 19 articles with 23,960 subjects, and the publication time ranged from 2006 to 2020. Among which, 12 articles were published in China, 3 in South Korea, 1 in the United States, Italy, Israel, and Turkey respectively (Table 1).
The relationship between serum TSH and BMD in postmenopausal women based on the Pearson correlation coefficient
The effect sizes were all Pearson correlation coefficients. The value of the correlation coefficient r in the previous studies was converted by the above-mentioned formula, and shown in Table 2.
We found that TSH was positively correlated with BMD, Fisher’ Z = 0.16, 95% CI (0.00, 0.32), Z = 1.98, P = 0.05 (Fig. 2). The final combined effect value r of TSH and BMD was 0.158.
BMD comparison among TSH groups with different levels in postmenopausal women with normal thyroid function
In original studies, the TSH was trisected according to the Tri -sectional quantiles, namely low, medium, and high. The cutoff value was incorporated into the previous group. We took the middle-level TSH group was used as the control in this study, to analyze the difference in BMD between the high-level TSH group and the low-level TSH group. The BMD of the high-level TSH group was higher than that of the control group, with SMD of 0.22, 95% CI (0.08, 0.35), P = 0.001. The BMD of the low-level TSH group was statistically lower than the control group, SMD at -0.31, 95% CI (-0.44, -0.18), P < 0.001 (Fig. 3).
The relationship between TSH and osteoporosis
Multivariate logistic regression could determine the frequency of osteoporosis in different groups with different TSH levels after adjusting confounding factors (age, BMI, BMD, usage of anti-osteoporosis drugs), combine the effect size OR included in the article.
We found that the risk of osteoporosis for low level TSH was 1.76 times, 95% CI (1.27, 2.45) of that high level TSH (Fig. 4). This indicates that low level TSH will increase the dependence of OP.
Results of subgroup analysis
A subgroup analysis of the source-based research subjects with OR as the measurement index, found that low level TSH group in the community facing higher risk of osteoporosis, OR = 1.89, 95%CI (1.43, 2.49), P < 0.01. In addition, whether anti-osteoporosis drugs were taken or not, low level TSH increased the risk of osteoporosis, OR at [1.84, 95%CI (1.05, 3.22), P = 0.03] and [1.74, 95%CI (1.08, 2.82), P = 0.02] respectively. The results of subgroup analysis of studies with the Pearson correlation coefficient as the outcome index could be found from Table 3. The subjects took calcium and other anti-osteoporosis drugs, summary Fisher’ Z = 0.14, 95%CI (0.02, 0.26), P = 0.03, which could affect the relationship between TSH and BMD. BMD detection site, whether the subjects suffered diabetes or not etc, had no influence on the relationship between TSH and BMD (Table 4).
The dose-response relationship between different TSH levels and osteoporosis
It could be seen in additional file 4 that OR of different TSH levels and OP, including 5 research [12, 16, 17, 21, 27] to reflect the dose-response relationship, each study used the high-level group as a control to analyzed the risk of osteoporosis in the different levels of TSH group within the normal range. The TSH level in the table was the average level. We employed Stata12.0 software to draw a dose-response diagram.
According to the dose-response relationship, we knew that even when TSH was within the normal range, TSH = 2.5mIU/L, the risk of osteoporosis kept in a high level. When it was lower or higher than 2.5mIU/L, the development of OP could be diminished, and the risk of osteoporosis gradually decreased with the increasing TSH level (Fig. 5).
Sensitivity analysis
After eliminating included articles one by one, it was found that with Wang Yi [9] Summary Fisher’ Z value at -2.65, and 95% CI (-2.84, -2.46), the conclusion of the study was totally opposite. Therefore, this article was excluded in the subsequent analysis (Additional file 5). In the research of the relationship between TSH and osteoporosis based on the OR value, the results were relatively stable after screening each studies (Fig. 6).
Publication bias
Publication bias was assessed using Egger’s test. Taking effect as the publication bias of Pearson correlation coefficient, it can be seen that the overall sample size included in the study was large and bilateral symmetric, and the publication bias with effect size as OR value is found to be P = 0.120, 95%CI (-0.663, 4.745). Therefore, no single article appears to influence the combined results (Fig. 7).
Discussion
Overt hypothyroidism is known to lower bone turnover by reducing both osteoclastic bone resorption and osteoblastic activity. These changes in bone metabolism would result in an increase in bone mineralization [28]. It is reported that the lifetime risk of osteoporosis in women is 40%-50% [29]. There were about 30% postmenopausal women who had osteoporosis in China [30]. However the relationship between serum TSH and BMD in postmenopausal women was still controversial, this study conducted meta-analysis and found that postmenopausal women’ s serum TSH was positively correlated with BMD, r = 0.158. The risk of osteoporosis in postmenopausal women with low-level TSH was 1.76 times of those with high-level TSH, 95% CI (1.27, 2.45). The dose-response relationship showed that when TSH was above 2.5mIu/L, the incidence of osteoporosis tended to be decreased. These results provided a theoretical basis for the prevention and treatment of osteoporosis in postmenopausal women.
Studies have demonstrated that serum TSH acts independently from thyroid hormone in bone metabolism. Wang Jiadan et al. [22]. found that the fluctuation of TSH level in the reference range in postmenopausal women with normal thyroid function may have a certain impact on the BMD of femoral neck, total hip and ward triangle. In addition, the TSH level may be an independent influencing factor of BMD in femoral neck and ward triangle, and those with low TSH level have a higher risk of osteopenia. TSH gets involved in bone turnover mainly through the following aspects: Firstly, TSH can promote osteoblast to secrete OPG, which can competitively inhibit the binding of RANK and RANKL, thus curbing the differentiation of osteoclast precursor cells into osteoclast. Secondly, TSH can activate protein kinase C6 in osteoblast to up-regulate frizzled and Wnt5a in non-canonical pathways, and induce osteoblast differentiation [31], which leaded to increasing level of BMD. This is consistent with the changing trend of TSH and BMD in the study of Wang Xiaodong [32]. The dose-response relationship showed that if the TSH level was below 2.5mIu/L, the risk of osteoporosis gradually increased, but TSH was up to 2.5mIu/L, it decreases with the increase of TSH level. BMD of the high-level TSH group was higher than that of the low-level TSH group, comparing with the BMD of the medium-level TSH group. It demonstrated that TSH was elevated when the BMD increased. Thirdly, TSH inhibits TNF-α and the proliferation and differentiation of osteoclast by binding to TSHR expressed on the surface of osteoclast [31]. In addition, TSH inhibits the binding of RANK and RANKL by directly inhibiting RANKL [33], thereby containing osteoclast precursor cells differentiate into osteoclast and curbing the formation of osteoclast [34] (Fig. 8), and reducing bone resorption.
From the Subgroup analysis it was found that the low-level TSH population from the community had a higher rate of osteoporosis than the patients from hospitals. Whether the anti-osteoporosis drugs were taken or not usually had no influence on the heterogeneity. According to subgroup analysis results targeting the different body part of BMD measurement and whether the subjects suffer from diabetes, we found that the relationship between TSH and BMD constant.
This study has a few limitations. First is that the articles included in this study are from cross-sectional survey and only conducted one measurement of TSH and BMD. Second, the value of BMD is not continuously measured as TSH changes. It will be more convincing that TSH and BMD be measured at a certain interval with multiple measurements. However, the concerned studies were tested for publication bias, and the results illustrated that there was no bias. Sensitivity analysis indicated that Wang Yi’s article [8] was highly sensitive, so it was excluded in the analysis. In future, the controlled trial should be conducted to clarify the relationship between TSH and BMD in postmenopausal women. At the same time, in vitro and in vivo experiments ought to be carried out to further explore how TSH promotes the secretion of OPG by osteoblast, and the specific signaling pathways and molecules that play a role in inhibiting the proliferation and differentiation of osteoclast.
Conclusions
In summary, the serum TSH in the normal range of postmenopausal women was positively correlated with BMD, and high level (> 2.5mIu/L) within the normal range is helpful to decrease the risk of osteoporosis in postmenopausal women.
Data Availability
Data will be available upon request from the corresponding author.
Abbreviations
- BMD:
-
bone mineral density
- OP:
-
osteoporosis
- TSH:
-
thyroid-stimulating hormone
- OR:
-
Odds ratio
References
Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;6(7):CD000333.
Mukherjee K, Chattopadhyay N. Pharmacological inhibition of cathepsin K: a promising novel approach for postmenopausal osteoporosis therapy[J]. Biochem Pharmacol. 2016;117:10–9.
Qiuguixing P. Huzhenming, etal. Guidelines for diagnosis and treatment of osteoporotic fractures in China principles of diagnosis and treatment of osteoporotic fractures[J]. Heilongjiang Sci. 2018;9(2):85–8.
Black DM, Rosen CJ. Clinical practice postmenopausal osteoporosis. N Engl J Med. 2016;374:254–62.
Luohuasong P. Liukebin, Yiyang, Chenliaobin. Literature review of osteoporosis and its related risk factors[J]. orthopedics. 2020;11(4):348–52.
Guohuaping Y, Chenwenhua, Yubo Q. Analysis of risk factors and preventive measures for postmenopausal osteoporosis[J]. Chin J Rehabilitation Med. 2011;26(5):424–8.
Etsuko Abe RC, Marians W. Yu et al 8[J] Cell. 2003;115(2):151–62.
Cuixinjie F, Niufengxiu N, Wangyingchao S. Study on the correlation between thyroid stimulating hormone (TSH) and bone mineral density in postmenopausal women with type 2 diabetes with normal thyroid function[J]. Chin J Osteoporos. 2020;26(3):385–9.
Wangyi. The effect of serum thyroid-stimulating hormone level on bone mineral density in postmenopausal women with type 2 diabetes[J]. Shandong Med. 2018;58(29):71–3.
Yinfei. Study on the correlation between bone mineral density and serum thyroid-stimulating hormone levels in postmenopausal women with type 2 diabetes[D]. Chengde Medical College; 2016.
Tsiligianni I, Kocks J, Tzanakis N, et al. Factors that influences disease-specific quality of life or health status in patients with COPD: a systematic review and meta-analysis of Pearson corrlations [J]. Prim Care Respir J. 2011;20(3):257–68.
Jae KD, Ho KY, Jung-Min K, Kee SY. Kim Ghi Su. Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women[J]. Clin Endocrinol. 2006;64(1):86–90.
Morris Martha Savaria. The association between serum thyroid-stimulating hormone in its reference range and bone status in postmenopausal american women.[J]. Bone. 2007;40(4):1128–34.
Gherardo Mazziotti T, Porcelli I, Patelli PP, Vescovi A, Giustina. Serum TSH values and risk of vertebral fractures in euthyroid post-menopausal women with low bone mineral density[J]. Bone. 2010;46(3):747–51.
Lin JD, Pei D, Hsia TL et al. The relationship between thyroid function and bone Mineral Density in Euthyroid healthy subjects in Taiwan[J]. 2011, 36(1): 1–8.
Leader Avi AR, Heffez L, Michael C, Efrat S, David. Hermoni Doron. Thyrotropin levels within the lower normal range are associated with an increased risk of hip fractures in euthyroid women, but not men, over the age of 65 years.[J]. J Clin Endocrinol Metab. 2014;99(8):2665–73.
Noh H-M, Park YS, Lee J, Lee W. A cross-sectional study to examine the correlation between serum TSH levels and the osteoporosis of the lumbar spine in healthy women with normal thyroid function[J]. Osteoporos Int. 2015;26(3):997–1003.
Berrin Acar AC, Ozay OE, Ozay E, Okyay AR, Sisman. Dinc Ozaksoy. Evaluation of thyroid function status among postmenopausal women with and without osteoporosis[J]. Int J Gynecol Obstet. 2016;134(1):53–7.
Linmei. Fracture risk assessment of postmenopausal women with normal serum TSH[D]. Shanxi Medical University; 2016.
Ding B, Zhang Y, Li Q, et al. Low thyroid stimulating hormone levels are Associated with Low Bone Mineral density in femoral Neck in Elderly Women[J]. Arch Med Res. 2016;47(4):310–4.
Lee SJ, Young KKyoungM et al. Low Normal TSH levels are Associated with Impaired BMD and Hip Geometry in the Elderly.[J]. Aging and disease, 2016, 7(6): 734–743.
Wangjiadan Z, Shilixin, Pengnianchun Z. The effect of physiological variation of thyroid-stimulating hormone on bone mineral density and osteoporosis in postmenopausal women[J]. Chin Gen Pract. 2017;20(8):907–11.
Niufengxiu. Study on the correlation between TSH level and bone metabolism in postmenopausal diabetic patients with normal thyroid function[D]. Qingdao University; 2018.
Qinliping L, Tangxulei, et al. Study on the correlation between the normal range of female thyroid-stimulating hormone levels and bone metabolism[J]. Chin J Osteoporos. 2018;24(9):1136–40.
Gaosaisai X. Correlation analysis between bone mineral density and serum thyroid-stimulating hormone levels in postmenopausal women with type 2 diabetes[J]. Chin Gen Pract. 2019;17(11):1853–5.
Zhanglihong Y, Tianzhufang L. Study on the relationship between thyroid stimulating hormone and osteoporosis in patients with type 2 diabetes[J]. Chin J Osteoporos. 2019;25(1):79–84.
Chenqingling P, Shilixin Z, Zhangmiao H. Study on the relationship between the level of thyroid-stimulating hormone and osteoporotic fracture in postmenopausal women[J]. Chin J Osteoporos. 2019;25(6):783–8.
Alessandro P. Delitala,Angelo Scuteri,Carlo Doria. Thyroid hormone Diseases and Osteoporosis[J]. J Clin Med,2020,9(4).
Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(Suppl 2):3–S7.
Ningweiqing Y, Wujie, Shencaie Y. Analysis of osteoporosis and related factors in postmenopausal women in Suzhou[J]. Jiangsu Med. 2011;37(18):2157–9.
Chenmiaomiao, Tongxishuai L. Research progress on the regulation of osteoclast differentiation by autophagy mediated by MAPK signaling pathway[J]. Adv Anim Med. 2020;41(2):92–7.
Wangxiaodong. The relationship between thyroid hormones, serum sex hormones and bone metabolism indexes in postmenopausal patients with hyperthyroidism[J]. Clin Med Res Pract. 2019;4(13):101–2.
Zhuyuqing SunlinThyroid. Stimulating hormone and bone Metabolism[J]. Chin Med J. 2019;21(2):315–8.
Dumic-Cule I, Draca N, Luetic A, et al. TSH prevents bone resorption and with Calcitriol synergistically stimulates bone formation in rats with low levels of calciotropic Hormones[J]. Horm Metab Res. 2014;46(5):305–12.
Acknowledgements
I would like to thank Professor Shugang Li and Yinfei Hu for their guidance in this study and thank our research team for their selfless help. This work was supported by Beijing High Level Public Health Technical Talents Training Plan Key Discipline Member-02–44, the National Natural Foundation of China (No: 72061137007) and the National Health Commission (Grant No. 2020YB06) of China.
Funding
This work was supported by Beijing High Level Public Health Technical Talents Training Plan Key Discipline Member-02–44, the National Natural Foundation of China (No: 72061137007) and the National Health Commission (Grant No. 2020YB06) of China.
Author information
Authors and Affiliations
Contributions
ZXL, LM, LSG and HYF were involved in the conception and design of the study. ZXL was responsible for writing the article and LM, LSG and HYF, Xinying Dong, Fen Liu provided important suggestions and opinions in the process of revising the article. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, X., Li, M., Dong, X. et al. A systematic review of the relationship between normal range of serum thyroid-stimulating hormone and bone mineral density in the postmenopausal women. BMC Women's Health 23, 358 (2023). https://doi.org/10.1186/s12905-023-02488-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02488-9