Abstract
Objective
To investigate the human papilloma virus (HPV) infection status, main subtypes and age distribution characteristics of women in the Putuo area of Shanghai.
Methods
A total of 13,936 subjects were enrolled in this study. These subjects were 15–89 years old, with a mean age of 41.68. Real-time fluorescence quantitative polymerase chain reaction technology was used to detect 21 types of HPV.
Results
A total of 2,500 subjects with HPV infections were detected in 13,936 cervical exfoliated cell specimens (total infection rate 17.9%). There were 15 people aged below 20,486 people aged 21-30,876 people aged 31-40,484 people aged 41–50, 338 people aged 51–60, and 301 people aged > 60. In total, 1,893 (75.7%) subjects had a single type of HPV infection, 424 (16.9%) had a double infection, and 183 had triple or more infections (7.4%). The top 6 subtypes of HPV infection in the Shanghai Putuo District were HPV 52 (3.81%), HPV 58 (2.46%), HPV 16 (2.43%), HPV 53 (2.30%), HPV 81 (1.74%) and HPV 39 (1.5%). The number of high-risk HPV infections was 1,978, and the total infection rate was 14.19%. The number of intermediate-risk HPV infections was 578, and the total infection rate was 4.15%. The number of low-risk HPV infections was 338, and the total infection rate was 2.43%.
Conclusion
The top 3 populations with HPV infection rates in the Putuo District, Shanghai, were ≤ 20 years old, older than 60, and 21–30 years old. The infection rate of HPV in cervical outpatient clinics was significantly higher than that of other departments. The 9-valent vaccine is recommended for HPV vaccination in this area.
Similar content being viewed by others
Introduction
Human papilloma virus (HPV), belonging to the papilloma vacuolar virus, is a small covalent double-stranded circular DNA virus mainly present in human skin, mucosa and female cervical epithelial cells. More than 200 kinds of HPV have been found [1,2,3]. Studies have shown that long-term repeated infection of certain subtypes of HPV is a risk factor for cervical cancer development in women. According to the World Health Organization (WHO), there are about 500,000 new cervical cancer cases annually. Cervical cancer is a common malignant tumour in the female reproductive tract and is ranked fourth in cancer mortality rate and second in female cancer [4,5,6,7]. About 80,000 women in China die from cervical cancer every year, so screening for HPV is necessary. In the face of these public health challenges, it is necessary to strengthen the management of medical resources and build a comprehensive system plan to promote the preparedness of communities and hospitals for diseases, so as to effectively curb the spread of these diseases [8,9,10].
At present, laboratory testing methods mainly include nucleic acid testing, cytology testing, pathological histology testing, and so on. The current HPV vaccination campaign is also in full swing. Some experts also believe that the HPV subtype infection situation and the region’s age factors should be considered for HPV vaccination. Among them, the HPV high-risk type tends to cause intraepithelial neoplasia of cervical cells. In contrast, the HPV low-risk type of infection causes genital tract warts. The HPV high-risk types include HPV 16, HPV 18, HPV 31, HPV 33, HPV 35, HPV 39, HPV 45, HPV 51, HPV 52, HPV 56, HPV 58, HPV 59, and HPV 68, five intermediate-risk types including HPV 26, HPV 66, HPV 53, HPV 73, and HPV 82, and three low-risk types including HPV 6, HPV 11, and HPV 81 [11]. However, there remains uncertainty regarding the prevalence of HPV infection and cervical risk in China. It is necessary to provide clinical evidence for the prevention of HPV infection and cervical cancer.
In this study, with patients in the department of gynaecology, the cervical department, the obstetrical department, and the physical examination department in the Putuo District, Shanghai, as study subjects, 21 subtypes of HPV infection were tested, using real-time quantitative polymerase chain reaction (Polymerase Chain Reaction, PCR) technology, aimed to understand the epidemiological characteristics of HPV in women in Shanghai in detail, analyse and evaluate the pathogenic risk of cervical lesions of different high-risk or suspected high-risk HPV, and provide a reference for developing cervical cancer screening strategies and the research, development and distribution of HPV vaccines suitable for China's national conditions.
Subjects and methods
Study design and subjects
This study is a retrospective, single-center study. A total of 13,936 females were selected to voluntarily undergo HPV examination in the departments of gynaecology, cervical, obstetrical and physical examination of the Shanghai Putuo District Maternal and Child Health Hospital from June 2021 to June 2022.
Research methods
Sample collection
The study subjects met the requirements: (1) the non-menstrual period; (2) Intravaginal irrigation and medication should not be performed 24 h before sampling. Cervical exfoliated cells were collected by routine gynaecological examination with a vaginal speculum to enlarge the vagina and to rotate the cervical sampler brush clockwise or counter clockwise for three to four laps. After sampling, it was placed in the storage solution of cervical exfoliated cell samples and sent to the specimen receiving laboratory.
Instruments and reagents
For HPV testing, an HPV typing testing kit (Jiangsu Shuoshi Biotechnology Co., Ltd., Taizhou, China, JC80301) was used. The SLAN-96P fluorescence quantitative PCR instrument (Shanghai Hongshi Medical Technology Co., LTD., Shanghai, China) was used for PCR analysis. HPV detection and typing method: Real-time quantitative polymerase chain reaction (Polymerase Chain Reaction, PCR) technology was used to target the human papillomavirus genome L1 region, 21 subform-specific primers and probes were designed, and the corresponding subtypes were marked with FAM, HEX and ROX, respectively. The probes were oligonucleotides, including the 5′-end reporter dye and the 3′-end quencher dye. During PCR amplification, when the probe is complete, the fluorescence emitted from the reporter dye is absorbed by the quencher dye due to its proximity to the reporter dye and emits no fluorescence signal. When the primer was extended, the probe bound to the template was cut off by the Taq enzyme (5′ → 3′ exonuclease activity). The reporter dye was separated from the quencher dye to produce the fluorescence signal. The quantitative PCR instrument automatically drew the real-time amplification curve according to the detected fluorescence signal to realise the qualitative detection of human papillomavirus on the nucleic acid level. At the same time, reference genes can be used to monitor and exclude false negatives due to instrument failure, reagent factors, improper manipulation, or inhibitors in the sample. The instrument automatically saves the results after the reaction. The baseline start point, endpoint, and threshold can be adjusted according to the analysed image. The result can also be automatically read by the instrument (default start cycle 6, stop cycle 12, and threshold 0.12). Click Analysis to automatically obtain the analysis results and find the results in the same interface.
Quality control
Positive plasmids and blank controls were used to monitor the amplification reactions during the amplification process. The experiment participated in the Shanghai temporary inspection centre room quality control as a laboratory quality assurance. Blank control: No typical S-type amplification curve is shown. Positive control: the HPV 21 subtypes and the reference gene test showed a typical S-type amplification curve and a CT value ≤ 30. Reference gene: The FAM channel amplification curve in group H has a typical S-type curve and CT ≤ 36.7, otherwise, it is related to errors caused by sampling, transportation, preservation conditions and experimental operation. The above requirements should be met simultaneously in the same experiment. Otherwise, the qualitative results of this experiment were invalid.
Statistical methods
Statistical analysis was performed using SPSS17.0 (IBM. Corp, Silicon Valley, CA, USA). Measurement data were tested for normality using the Shapiro–Wilk test. Data following normal distribution were described by the mean ± standard deviation. The count data were statistically described by number (%). The rate of HPV infection was compared using the chi-square test. P < 0.05 was considered a statistically significant difference. The sample size was calculated using the PASS software (version 15, NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/pass) with the module of confidence interval for one proportion. A sample size of 715 produces a two-sided 95% confidence interval with a width equal to 0.060 when the sample proportion is 0.200.
Results
General data and HPV infection status of the study subjects
A total of 13,936 study subjects were included, aged 15–89, with a mean age of 41.7 ± 12.3 years. Subjects aged 31–40 accounted for the highest proportion (36.17%). All patients were from urban areas and had experienced sexual activity but were not HPV vaccinated. Most patients were married (89.16%) and were educated to the level of junior high school or above (82.71%). In addition to the presence or absence of HPV infection, some patients were infected with other sexually transmitted diseases, such as Ureaplasma urealyticum (47.93%) (Table 1).
Infection status of HPV-positive patients
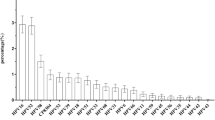
A total of 2,500 HPV infections were detected in 13,936 HPV screening specimens, and the total positive rate was 17.9%, among which the highest positive rate was respectively: HPV 52 (3.81%), HPV 58 (2.46%), HPV 16 (2.43%), HPV 53 (2.30%), HPV 81 (1.74%) and HPV 39 (1.52) (Table 2).
Comparison of HPV detection among all ages
A total of 2,500 individuals with HPV infection were found among the 13,936 cervical detached cell specimens, for an overall infection rate of 17.9%. The 13,936 patients were classified into six age groups: patients under the age of 20, patients between the ages of 21 and 30, patients between the ages of 31 and 40, patients between the ages of 41 and 50, patients between the ages of 51 and 60, and patients older than 60. Using quantitative PCR, the infected population was segmented into 15 patients under the age of 20 (infection rate: 33.33%), 486 patients between the ages of 21 and 30, 876 patients between the ages of 31 and 40, 484 patients between the ages of 41 and 50, 338 patients between the ages of 51 and 60, and 301 patients over the age of 60 (infection rate: 20.70%). The infection rates among the age categories varied significantly (Table 3).
Analysis of infection with different HPV subtypes
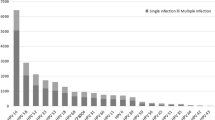
Of the 2,500 HPV-positive specimens, 1,893 (75.7%) were single infections, 424 (16.9%) were double infections, and 183 (7.4%) were triple or more infections (Fig. 1). For 2,500 HPV-positive specimens, the number of infections was 399 (14.08%) in the outpatient obstetric department, 1,334 (39.46%) in the outpatient cervical department, 650 (9.55%) in the outpatient department of gynaecology and 117 (12.72%) in the outpatient physical examination department (Fig. 2). There were statistically significant differences among the various departments (X2 = 1433.6, P < 0.05). Of all the 13,936 specimens, the numbers of high-risk, moderate risk, and low-risk HPV infections were 1,978 (14.19%), 578 (4.15%), and 338 (2.43%), respectively.
Discussion
HPV infection and age showed a "V" type distribution
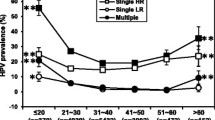
This study shows that the top three ages with a high incidence of HPV are ≤ 20 years old, > 60 years old and 21–30 years old. Therefore, we should not only vaccinate these people with the HPV vaccine in advance but also pay attention to the importance of reproductive health and HPV vaccination in our daily science popularisation work. Investigating the prevalence of HPV infection according to different age populations is of great importance to achieve the precision prevention and establish appropriate therapeutic strategies [12, 13]. For women aged > 60 years, the HPV infection rate is also high, so different management for HPV is targeted at different populations. Women aged > 60 can be diagnosed primarily with HPV screening combined with thin-cytologic test (TCT) results.
HPV virus infection rate and subtype infection situation
Our findings provided further insights into the prevalence of HPV infections according to different subtypes, which was useful to establish the epidemiological distribution pattern of HPV in the local area and provide guidance for secondary and tertiary prevention. In this study, the total infection rate of 13,936 women was 17.9%, lower than the 22.6% reported in the Beijing area [14] and lower than the 26.26% reported in Shaanxi province [15]. This finding indicated that the distribution pattern of HPV infection might exhibit specific characteristics from different regions and living habits. The top three subtypes of HPV infection in the Putuo District were HPV 52 (3.8%), HPV 58 (2.5), and HPV 16 (2.4%), which are consistent with the top three subtypes reported by Ming Chunyan et al. [16]. These subtypes of HPV infections might be the focus of prevention and treatment of cervical cancer in the future. Previous studies have shown that persistent infection of the HPV 16 subtype is closely related to cervical cancer, and HPV 16, HPV 52, and HPV 58 cause specific cancer in Chinese women, so secondary prevention against cervical cancer is still essential in regional screening [17,18,19]. Because chronic cervicitis leads to cervical cancer under chronic HPV virus repeated infection, long-term follow-up is still necessary [20, 21].
HPV 52 had a higher incidence of cervical lesions compared to HPV 16, HPV 53, and HPV 58. The findings suggested that HPV 56, HPV 58, and HPV 52 were associated with an increased incidence of cervical lesions compared to HPV 16, and that HPV 53/58/52 infection individuals may also be sent for colposcopy in addition to HPV 16 and 18. There are few related studies on other HR-HPV cancer-causing forces, and no unified cognition has been formed. Wang et al. [22] reported a higher detection rate of HSIL in HPV 16/18/31/33/52/58 infected persons after colposcopy. The conclusions of Sung et al. [23] also suggest that more aggressive action may be needed for HPV 52/58-infected patients.
The present study has some research limitations. First, the source of our sample is relatively limited, only for population screening in key departments, and cannot represent the screening results of the whole population, which may have some bias in the extrapolation of the results. Second, we have only analysed the infection status of HPV and have not yet focused on the relationship between HPV infection and its outcome shunt, which should be further deepened in future studies. Third, the generalizability of our results is limited due to our single-center design. The validity of this study would increase if the rural population was also included. Finally, we have not analysed the association between individual basic characteristics and HPV infection, and therefore, we will analyse the association in more depth in subsequent studies.
Conclusion
In conclusion, HPV infections in the Putuo area, Shanghai, is still at a high level, and the top-ranked HPV infection subtypes are HPV 52, HPV 58, HPV 16, and HPV 53. We should take more proactive measures against HPV 52/58/53 infection with the cervical cancer screening strategy, vaginal referral, and the future development of the HPV vaccine in China, as it serves as a reminder that the HPV vaccine vaccination is still the primary method of preventing and controlling cervical cancer at our primary level. Further studies are warranted to elucidate the mechanism behind the association with HPV infection and risk of cervical lesions in this area with a long-term follow-up.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
References
Xi LF, Schiffman M, Ke Y, Hughes JP, Galloway DA, He Z, et al. Type-dependent association between risk of cervical intraepithelial neoplasia and viral load of oncogenic human papillomavirus types other than types 16 and 18. Int J Cancer. 2017;140(8):1747–56. https://doi.org/10.1002/ijc.30594. (Epub 2017 Jan 24).
Jin R, Li HF. The effect of persistent high-risk HPV infection on the progression of cervical precancerous lesions. Matern Child Health Care China. 2020;35(3):406–9. https://doi.org/10.19829/j.zgfybj-issn.1001-4411.2020.03.006.
Gupta G, Giannino V, Rishi N, Glueck R. Immunogenicity of next-generation HPV vaccines in non-human primates: measles-vectored HPV vaccine versus Pichia pastoris recombinant protein vaccine. Vaccine. 2016;34(39):4724–31. https://doi.org/10.1016/j.vaccine.2016.07.051. (Epub 2016 Aug 11).
Ward JM, Schmalenberg K, Antonishyn NA, Hambleton IR, Blackman EL, Levett PN, et al. Human papillomavirus genotype distribution in cervical samples among vaccine naïve Barbados women. Cancer Causes Control. 2017;28(11):1323–32. https://doi.org/10.1007/s10552-017-0959-y.
Asciutto KC, Henningsson AJ, Borgfeldt H, Darlin L, Borgfeldt C. Vaginal and urine self-sampling compared to cervical sampling for HPV-testing with the cobas 4800 HPV test. Anticancer Res. 2017;37(8):4183–7. https://doi.org/10.21873/anticanres.11807.
de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology. 2013;445(1–2):2–10. https://doi.org/10.1016/j.virol.2013.04.023.
Baedyananda F, Chaiwongkot A, Bhattarakosol P. Elevated HPV16 E1 expression is associated with cervical cancer progression. Intervirology. 2017;60(5):171–80. https://doi.org/10.1159/000487048.
Khademipour G, Nakhaee N, Anari SMS, Sadeghi M, Ebrahimnejad H, Sheikhbardsiri H. Crowd simulations and determining the critical density point of emergency situations. Disaster Med Public Health Prep. 2017;11(6):674–80. https://doi.org/10.1017/dmp.2017.7.
Sheikhbardsiri H, Khademipour G, Davarani ER, Tavan A, Amiri H, Sahebi A. Response capability of hospitals to an incident caused by mass gatherings in southeast Iran. Injury. 2022;53(5):1722–6. https://doi.org/10.1016/j.injury.2021.12.055.
Nobakht S, Shirdel A, Molavi-Taleghani Y, Doustmohammadi MM, Sheikhbardsiri H. Human resources for health: a narrative review of adequacy and distribution of clinical and nonclinical human resources in hospitals of Iran. Int J Health Plann Manage. 2018. https://doi.org/10.1002/hpm.2510.10.1002/hpm.2510.
Krashias G, Koptides D, Christodoulou C. HPV prevalence and type distribution in Cypriot women with cervical cytological abnormalities. BMC Infect Dis. 2017;17(1):346. https://doi.org/10.1186/s12879-017-2439-0.
Molavi-Taleghani Y, Ebrahimpour H, Sheikhbardsiri H. A proactive risk assessment through healthcare failure mode and effect analysis in pediatric surgery department. J Compr Pediatr. 2020. https://doi.org/10.5812/compreped.56008.
Safi-Keykaleh M, Aliakbari F, Safarpour H, Safari M, Tahernejad M, Sheikhbardsiri H, et al. Prevalence of postpartum depression in women amid the COVID-19 pandemic: a systematic review and meta-analysis. Int J Gynaecol Obstet. 2022;157(2):240–7. https://doi.org/10.1002/ijgo.14129.
Wang HB, Zhang DQ, Zhao J. Analysis of female human papillomavirus infection in Haidian District, Beijing. J Mol Diagn Ther. 2020;12(10):1411–4. https://doi.org/10.3969/j.issn.1674-6929.2020.10.032.
Fan J, Li N, Zhang ZM, An RF, Li J, Zhao JN, et al. Distribution characteristics of human papillomavirus infection genotypes in cervix of women in Shaanxi Province[J]. China Matern Child Health Res. 2015;26(5):958–61. https://doi.org/10.3969/j.issn.1673-5293.2015.05.016.
Ming CY, Huang KL, Zhao MJ, Zhao J, Wang J, Du LJ. Analysis of high-risk HPV infection and its cervical lesions in women in Nanchong area. China Matern Child Health Res. 2022;33(4):115–9. https://doi.org/10.3969/j.issn.1673-5293.2022.04.021.
Wang L, Wang JH, Zhang J, Liu BB, Yu XJ, Zhu YL. Analyses of human papillomavirus infection and genotype distribution status among cervical cancer patients in northern Jiangsu Province and southern Anhui Province. Cancer Res Clin. 2021;33(12):933–7. https://doi.org/10.3760/cma.j.cn115355-20201230-00740.
Rohner E, Edelman C, Sanusi B, Schmitt JW, Baker A, Chesko K, et al. Extended HPV genotyping to compare HPV type distribution in self- and provider-collected samples for cervical cancer screening. Cancer Epidemiol Biomark Prev. 2020;29(12):2651–61. https://doi.org/10.1158/1055-9965.EPI-20-0674.
Aro K, Nieminen P, Louvanto K, Jakobsson M, Virtanen S, Lehtinen M, et al. Age-specific HPV type distribution in high-grade cervical disease in screened and unvaccinated women. Gynecol Oncol. 2019;154(2):354–9. https://doi.org/10.1016/j.ygyno.2019.05.024.
Zhang J, Cheng K, Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch Gynecol Obstet. 2020;302(6):1329–37. https://doi.org/10.1007/s00404-020-05787-w.
Dong L, Wang MZ, Zhao XL, Feng RM, Hu SY, Zhang Q, et al. Human papillomavirus viral load as a useful triage tool for non-16/18 high-risk human papillomavirus positive women: a prospective screening cohort study. Gynecol Oncol. 2018;148(1):103–10. https://doi.org/10.1016/j.ygyno.2017.11.016.
Wang Z, Gu Y, Wang H, Chen JY, Zheng YW, Cui BX, et al. Distribution of cervical lesions in high-risk HPV (hr-HPV) positive women with ASC-US: a retrospective single-center study in China. Virol J. 2020;17(1):185. https://doi.org/10.1186/s12985-020-01455-2.
Sung YE, Ki EY, Lee YS, Hur SY, Lee A, Park JS. Can human papillomavirus (HPV) genotyping classify non-16/18 high-risk HPV infection by risk stratification? J Gynecol Oncol. 2016;27(6):e56. https://doi.org/10.3802/jgo.2016.27.e56.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LHP have made substantial contributions to conception and design, LHP acquisition of data, analysis and interpretation of data; LHP have been involved in drafting the manuscript and revising it critically for important intellectual content; LHP have given final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the declaration of Helsinki.This study was conducted with approval from the Ethics Committee of Putuo District Maternal and Child Health Hospital (Approval Number: NO.PFYLL-2021003). Written informed consent was obtained from all participants and their parent and/or legal guardian.
Consent for publication
Not applicable.
Competing interests
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luan, H. Human papilloma virus infection and its associated risk for cervical lesions: a cross-sectional study in Putuo area of Shanghai, China. BMC Women's Health 23, 28 (2023). https://doi.org/10.1186/s12905-023-02166-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-023-02166-w