Abstract
Background
Mastectomy is the first-line treatment approach for more than 90% of breast cancer patients. The numerous physical impairments associated with this surgical procedure negatively impact the patient’s quality of life. To date, rehabilitation resources available for breast cancer patients undergoing mastectomy within the institutions affiliated to the Centre intégré universitaire de soins de santé et de services sociaux de la Mauricie-et-du-Centre-du-Québec (CIUSSS-MCQ) are lacking and do not always seem to reflect the particularities of breast cancer care pathways. The purpose of this review was to identify and describe the conservative interventions and the clinical outcome measures used in the perioperative physical rehabilitation of women with breast cancer who are awaiting or have undergone mastectomy. We also aimed to report on the barriers and facilitators to study participation and completion.
Methods
MEDLINE, CINAHL, and the Cochrane Library were searched from inception to January 2021, and we updated the search on July 11, 2022. We included peer-reviewed English and French literature with quantitative designs, describing conservative interventions and clinical outcome measures used within rehabilitation programs designed for women who were awaiting or had undergone mastectomy. Paired reviewers independently reviewed all citations and articles using a two-phase screening process and independently extracted the data.
Results
Of the 6080 articles identified, 57 met the inclusion criteria. Most interventions were multimodal, which combined exercise with patient education, manual therapy, and/or lymphatic drainage. The most frequently used objective measures of physical function were shoulder range of motion, muscle strength, and signs of lymphedema. In contrast, the primary patient-reported outcome measures were quality of life, shoulder function, and pain. Undergoing another breast surgery, death, and cancer recurrence were the most reported barriers to study completion.
Conclusion
This scoping review reports on the heterogeneity and wide range of conservative interventions and clinical outcome measures used in the physical rehabilitation of breast cancer patients who had undergone or were scheduled to undergo mastectomy. Tailoring interventions to breast cancer patients’ needs and promoting outpatient rehabilitation interventions appear to be better suited to the particularities of breast cancer care pathways. Further research is needed to better identify barriers and facilitators to study participation and completion.
Similar content being viewed by others
Background
Breast cancer is a malignant tumor with the second highest incidence rate among females worldwide [1]. In 2020, breast cancer cases accounted for one in four new cancer diagnoses among Canadian women [1]. Implementation of a biennial population-based mammography screening program in 1998 [2], along with the improvement of surgical techniques [3] has fortunately contributed to a significant decrease in breast cancer mortality rates in Canada over the last twenty years [4]. Specifically in Quebec, over 90% of breast cancer patients in the early 2000s were diagnosed with an in situ breast tumor (stage 0) or a stage I or II disease [5]. Early detection of lower histological grade cancer significantly improves breast cancer patients’ prognosis, allowing treatment strategies to be initiated sooner, thus reducing the risk of disease progression [5]. In 2003, patients diagnosed with a stage I or II breast tumor showed a 5-year survival rate of 98.1% and 89.2%, respectively, while this number dropped to 10.5% for patients with a stage IV disease [5]. Although breast cancer patients may now benefit from a longer life expectancy, this is not without consequences for those women, who will still need to undergo a series of therapeutic interventions whose physical, psychological, and socio-economic effects are substantial [6].
Mastectomy (i.e., surgery to remove part of or all the breast) represents the first-line treatment approach for more than 90% of breast cancer patients [7]. Physical impairments associated with this surgical procedure are numerous (e.g., loss of shoulder range of motion (ROM), pain, lymphedema, and muscle weakness) [8], leading to limitations in activities of daily living, which negatively impacts the patient’s quality of life [9, 10]. Several studies aimed to develop effective interventions to support breast cancer patients dealing with musculoskeletal adverse events (AEs) resulting from a mastectomy. A systematic review published in 2015 by De Groef et al. [11] confirmed the effectiveness of multimodal physical therapy (i.e., stretching exercises combined with general active exercises) to treat upper limb impairments after breast cancer treatments. Another systematic review published in 2019 by Ribeiro et al. [12] concluded that ROM and upper extremity strengthening exercises effectively improve shoulder ROM in patients who had undergone breast surgery. However, when comparing the 15 randomized controlled studies included in this review, the rehabilitation interventions described were found to be highly heterogeneous [12]. Although there seems to be no consensus as to which parameters should be chosen to promote optimal postoperative recovery for breast cancer patients, the use of self-management strategies in cancer patients is widely emphasized in the literature for its perceived benefits on patients’ quality of life and ability to manage treatment-related symptoms, besides promoting better utilization of health care and services [13, 14].
To date, there are limited rehabilitation resources available for breast cancer patients undergoing mastectomy within the institutions of the Centre intégré universitaire de santé et de services sociaux de la Mauricie-et-du-Centre du Québec (CIUSSS-MCQ). Previously published systematic reviews certainly provide important insights regarding the rehabilitation of women who have undergone mastectomy for breast cancer, but these have focused primarily on interventions initiated in the early postoperative period, and targeted specific outcome measures. Consequently, to ensure that we will provide timely and comprehensive patient care for women undergoing mastectomy, we must first establish a more comprehensive portrait of perioperative rehabilitation interventions and current clinical outcome measures. It stands to reason that such understanding represents a prerequisite for developing interventions whose modalities will reflect patients’ needs and expectations and consider the particularities of breast cancer care pathways.
Therefore, this study aimed to identify the conservative interventions and the clinical outcome measures used as part of the perioperative physical rehabilitation of women diagnosed with breast cancer who plan to or have undergone mastectomy. As a secondary objective, we aimed to report on the barriers and facilitators to participating and completing these rehabilitation programs.
Methods
Study design
To address our broad research question, a scoping review was conducted based on the framework from Arksey and O’Malley [15] and Levac et al. [16]. This type of study allows us to report on the current state of knowledge in a research field and captures the breadth of information on a topic that has been widely studied and for which the available data are numerous and heterogeneous [17]. Consistent with this framework, we did not appraise the methodological quality of the included studies.
Identifying the research question
Our scoping review was guided by the following research question: What are the conservative interventions and clinical outcome measures used as part of the perioperative physical rehabilitation of women diagnosed with stage 0-III breast cancer who are awaiting or have undergone a mastectomy?
Identifying relevant studies
Data sources and searches
Our search strategy was developed by one of the authors (J.M.) and two coauthors (A.A.M., M.D.) subsequently cross-validated the search to ensure completeness of results. The search strategy was first developed in MEDLINE and then adapted to other bibliographic databases. Search terms included controlled vocabulary for each database and free text words for the key concepts of breast cancer, mastectomy, and rehabilitation (see Additional file 1 for full search strategy). In addition, reference lists from relevant articles and previously published systematic reviews were hand searched for any additional relevant studies. We initially searched MEDLINE, CINAHL, and Cochrane databases from inception to January 24, 2021, and updated the search on July 11, 2022. EndNote X9 was used to de-duplicate references electronically across all databases.
Study selection
Eligibility criteria
To be included, studies had to meet the following criteria: (1) be written in the English or French language; (2) were randomized controlled trials, quasi-randomized trials, cohort studies, secondary analysis, exploratory studies or systematic reviews (for reference purposes only); (3) focused on adult women (aged ≥ 18 years) who engaged in a physical rehabilitation intervention before or following any type of mastectomy (e.g., partial mastectomy or breast conserving surgery (BCS), lumpectomy, quadrantectomy, wide local excision, segmental mastectomy) for a stage 0-III breast cancer. Studies including participants that underwent a mastectomy combined with an axillary staging procedure (i.e., axillary sampling or sentinel lymph node biopsy) or a lymph node dissection (ALND) were also included, considering that these surgical interventions are in line with the Society of Surgical Oncology-American Society of Clinical Oncology (SSO-ASTRO) clinical practice guideline recommendations [18]. All included studies also had to match the following characteristics for physical rehabilitation interventions:
-
1.
Initiated within 3 months preceding or following the surgical intervention.
-
2.
Involved at least one active physical modality (i.e., the patient physically contributed to its own treatment), including but not limited to exercises, conditioning, yoga, Taiichi, and Pilates.
-
3.
Provided alone or in combination with other types of conservative interventions (e.g., patient education, manual therapy, manual lymphatic drainage (MLD), nutritional or psychological interventions).
Study exclusion criteria included: cross-sectional studies, case report and case series designs, study protocol, practice guidelines, letters, editorials, commentaries, unpublished manuscripts, books and book chapters, conference proceedings, cost analyses, meeting and conference abstracts, thesis and dissertations, non-systematic reviews, qualitative studies, laboratory studies and cadaveric or animal studies. Studies focusing on breast cancer survivors (i.e., patients who had completed all forms of cancer treatments), on patients with a stage IV disease, on managing or preventing the AEs of systemic treatments (i.e., chemotherapy, radiation, or hormonal therapy) rather than surgery, and studies who failed to provide enough methodological details (i.e., minimally a description of the intervention’s procedures and its initiation time) to enable interventions’ replication were also excluded.
Screening and agreement
A two-phase screening process was used to select eligible studies. In phase I screening, a pair of independent reviewers (J.M., C.D.) screened citation titles and abstracts to determine the eligibility of studies (categorizing studies as possibly relevant or irrelevant). In instances where eligibility could not be ensured due to limited information in the title/abstract, the citation was considered “possibly relevant’’ until a final decision could be made upon full text review. A pair of independent reviewers (J.M., N.L.) screened possibly relevant studies in full text during phase II screening to determine eligibility and reasons for exclusion were documented. Reviewers met to discuss disagreements and to reach consensus in both phases. An additional reviewer (A.A.M.) was involved if consensus could not be reached.
Data charting
Both reviewers (J.M., N.L.) extracted the following data (when available) from half of the eligible studies: (1) study description (first author, publication year and country of origin); (2) study population (sample size, cancer stage, surgery type and systemic treatment administered); (3) rehabilitation interventions provided (e.g., type, initiation, duration, frequency); (4) outcome measures and outcome validation information and (5) patients’ experience data (e.g., reasons for not completing the study or for declining to participate, adherence outcomes, postoperative complications, AEs). An evidence table was built (see Additional file 2: Table S1) using a Microsoft Word document. A third reviewer (M.D.) independently verified the extracted data to minimize error.
Data synthesis and analysis
A descriptive synthesis was conducted to provide details regarding the total number of studies kept for analysis, their authors and year of publication, country where they were conducted, study design, and study population. The summary of evidence table includes a brief description of conservative rehabilitation interventions identified as well as outcome measures used for each of them. Interventions’ procedures and data on barriers and facilitators to engagement in these interventions were summarized separately in Additional file 2: Tables S1 and S2. To answer our research question, our review findings were sorted by themes of interest: “conservative rehabilitation interventions,” “clinical outcome measures,” and “patients’ experience.”
Results
Descriptive synthesis
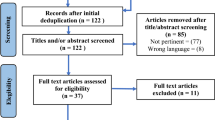
A total of 6068 articles were identified from the literature search, and twelve articles were retrieved from additional data sources. Following the removal of duplicates (n = 958), 5065 articles were excluded (see Fig. 1), bringing the total count to 57 papers, including 54 original studies.
Table 1 summarizes the key findings from the included articles. Most studies (39 of 57) were RCT [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57], five were controlled non randomized clinical trials [58,59,60,61,62,63], four were prospective cohort studies [61, 64,65,66], one was a retrospective cohort study [67], two was a case–control study [68, 69], four were quasi-experimental [70,71,72,73], one was a pilot study [74] and one was a feasibility study [75]. The studies originated from 22 countries distributed across 4 continents (i.e., Americas, Europe, Asia and Oceania), most of which were high income countries [19,20,21,22,23,24,25,26, 29, 31, 33,34,35,36, 39,40,41, 44,45,46,47, 50, 52,53,54, 56, 57, 59, 61,62,63,64,65,66,67,68,69, 71, 73,74,75] or upper-middle income countries [27, 28, 30, 32, 37, 38, 42, 43, 48, 49, 58, 60, 72], with only three studies conducted in lower-middle income countries [51, 55, 70]. The body of literature on this topic turned out to be quite recent, with 71.9% (41 of 57) of studies published between 2010 and 2022 and 22.8% (13 out of 57) between 2000 and 2009.
Participants
The studies’ sample size varied from 22 to 1217 participants, with participants’ mean age ranging from 44.1 to 63.4 years old. Most studies (34 out of 57) included women diagnosed with different breast cancer stages (i.e., 0-III), who underwent breast surgery combined with either an axillary staging procedure or ALND, and systemic treatments (i.e., chemotherapy, hormonotherapy, or radiotherapy). In 80.7% of the studies (46 out of 57), the study groups included patients who underwent BCS and those who underwent a total mastectomy. Therefore, no conclusions could be drawn as to whether the type of surgery might have an impact on clinical outcomes and on breast cancer patients' motivation to engage in and complete a rehabilitation intervention.
Conservative rehabilitation interventions
Four main modalities were identified amongst rehabilitation programs, which were consistent with exercises, patient education, MLD, and manual therapy. Exercises were part of every rehabilitation program, with 43.9% (25 out of 57) of these interventions being unimodal. Multimodal interventions were characterized by 2 to 4 modalities, the most common combinations being: (1) exercise and patient education (40.6%); (2) exercise and manual therapy (15.6%); (3) exercise, patient education, and manual therapy (15.6%); and (4) exercise, patient education, and MLD (12.5%). Nearly half of rehabilitation interventions (47.4%) were delivered using a mixed approach, initially performed under nursing staff or physical therapists' supervision and, in most instances, transitioned to a home-based intervention upon hospital discharge. Home-based interventions (15.8%) all consisted of exercises, which were either performed alone [38, 43, 68, 72], combined with patient education [36, 56, 64, 74] or with manual therapy [63]. Six studies reported implementing group interventions consisting solely of supervised exercise programs [30, 41, 49, 55, 60] or exercise combined with manual therapy [37].
Figure 2 illustrates the identified rehabilitation interventions’ timing, duration, and modalities. This graphical representation was constructed only for studies that clearly defined all three components. Looking at these studies (35 out of 57), we noted that 74.3% of interventions were initiated a few days to 4 weeks following surgery and went on for 2 to 24 weeks, while 3 interventions [21, 67, 73] lasted up to 12 months.
Initiation, duration and modalities characterizing rehabilitation interventions. aAmmitzboll, Kristina Kjaer et al., 2019 & Ammitzboll, Johanssen et al., 2019; bKilbreath et al. [33, 34] cOliveira et al. [58, 60]
 Blue: Exercises; Orange: Manual therapy; Yellow: Lymphatic drainage; Green: Education; White: Usual care
Blue: Exercises; Orange: Manual therapy; Yellow: Lymphatic drainage; Green: Education; White: Usual care
Preoperative rehabilitation interventions
Out of all included studies, 10 interventions [36, 48, 49, 51, 52, 61, 64, 69, 73, 75] were initiated before surgery, including eight that were pursued from 4 weeks to 12 months post-surgery. Only patient education and active shoulder ROM or aerobic exercises were implemented in the preoperative period. Six studies used these modalities as stand-alone, while 4 studies [48, 61, 64, 69, 73] combined them postoperatively with manual therapy or MLD. Educational strategies primarily focused on sharing information about postoperative complications, activity restrictions, prevention of lymphedema, infections or injuries, and explaining the upcoming surgical procedure.
Exercises
Types of exercises included in the rehabilitation programs are detailed in Fig. 3. Eleven types of exercises were identified, the most frequently reported being: (1) upper limb ROM exercises (77.2%); (2) stretching of shoulder muscles (45.6%); and (3) upper limb strengthening exercises (35.1%). Although a small proportion of studies (21.1%) suggested a single type of exercise, most built programs including 2–5 different types. Exercises targeting upper limb tissues and function were predominant. Fewer studies adopted a more global approach, providing aerobic exercises [21, 23, 52, 66, 71] or yoga [41, 55], as well as strengthening or stretching of the lower extremity [19,20,21] or neck muscles [28, 44, 57, 58, 60,61,62,63, 66, 72].
Patient education
Educational strategies were included in 42.1% (24 out of 57) of rehabilitation interventions identified. Prevention and lymphedema awareness, skincare routine, risks of postoperative complications, and physical activity or nutrition counseling were the cornerstones of these strategies. Nine studies also reported prescribing shoulder ROM limitations and activity restrictions (e.g., avoiding lifting, carrying heavier items, running, jumping, or other strenuous activities) up to 6 weeks following surgery [22, 32, 39, 51, 57] or upon surgical drains removal [36, 53, 54, 75].
MLD
Eight studies [26, 31, 42, 46, 48, 50, 58, 60] included MLD within their rehabilitation programs. Gentle pressure and circular massage were generally applied along the course of superficial lymph nodes lining the axillary region, the lateral aspect of the shoulder, the base of the neck, the chest region, and the affected and non-affected arm and hand. MLD was performed either by trained physical therapists or self-administered following supervised sessions. While most studies reported initiating this modality a few days following surgery without further indications, two studies [31, 48] described waiting for suture and surgical drain removal before proceeding.
Manual therapy
Thirteen studies incorporated manual therapy into their rehabilitation programs. This modality was always paired with exercises and, in some cases, complemented with MLD [26, 31, 46]. Passive scapular and shoulder joint mobilizations, scar tissue massage and passive shoulder muscle stretching performed by trained physical therapists [23, 26, 31, 32, 37, 46, 63, 69, 73] mainly characterized manual therapy. Two studies also included passive mobilizations of the elbow, wrist, and hand on the affected side [31, 44].
Reporting of interventions
Details of interventions’ components were extracted using the TIDieR checklist and guide [76] and are provided in Additional file 2: Table S2. Almost all studies (55 out of 57) reported more than 50% of TIDieR checklist items. Only 3 studies reported modifications to their protocol, and 17 out of 57 provided details regarding intervention adherence. Although 87.72% of studies described the intervention schedule, 15 studies did not specify the duration of interventions.
Clinical outcome measures
Three categories of outcome measures were used to report the effects of rehabilitation interventions on breast cancer patients undergoing mastectomy, including objective measures of physiological and physical function and patient self-reported outcome measures (PROMS). Figure 4 illustrates the outcomes investigated in each category and the measurement tools used for each. Thirty-three unique outcome measures (i.e., 15 physical, 15 PROMS, and 3 physiological) were used across studies, using 54 different measurement tools. Each study used a range of 1 to 7 outcomes, and most studies (37 out of 57) included outcomes from at least 2 of the 3 categories, all of which but one combined PROMS with objective measures of physical function. The most reported outcomes of physical function were shoulder ROM, muscle strength, and signs of lymphedema, measured by the goniometer, the dynamometer and arm circumference or volume, respectively. Quality of life (QoL), shoulder function, and pain were the PROMS most often reported. The European Organization for Research and Treatment of Cancer questionnaire (EORTC QLC C-30/BR23), the Disability of the Arm, Shoulder and Hand questionnaire, and the Visual Analogue Scale were the most frequently used outcome measures for these three domains. Three studies also investigated objective measures of physiological function, such as chest expansion [70], the forced expiratory volume in one second (FEV1) [70, 71], and the forced vital capacity (FVC) [71].
Outcome measures. Abd: Abduction; ADL: Activities of daily living; ALN: Axillary lymph nodes; BC: Breast Cancer; CCI: Comprehensive complication index; CR-10: Borg’s Category Scale for Ratings of Perceived Pain; CV: Cardiovascular; DASH: Disabilities of the Arm, Shoulder and Hand; DXA: Dual-energy X-ray absorptiometry; EORTC QLC: European Organization for Research and Treatment of Cancer quality of life questionnaire; FACT-B: Functional Assessment of Cancer Therapy—Breast; FACIT-f: Functional Assessment of Chronic Illness Therapy – Fatigue; FVC: Forced vital capacity; FEV1: Forced expiratory volume in one second; GARS: Groningen Activity Restriction Scale; HRQOL: Health-related quality of life ILMD: Interlimb mass difference; ISL: International Society of Lymphology; NRS: Numeric Rating Scale; PCS: Pain Catastrophizing Scale; PROMS: Patient-reported outcome measures; QoL: Quality of life; RM: Repetition Maximum; ROM: Range of motion; SDQ: Shoulder Disability Questionnaire; SF-36: 36-Item Short Form Health Survey; SGPALS: Saltin-Grimpy Physical Activity Level Scale; SIP: Sickness Impact Profile; TKS: Tampa Kinesiophobia Scale; UCLA: University of California at Los Angeles; ULDQ: Upper Limb Disability Questionnaire; VAS: Visual analogue scale; 6MWT: 6-Minute Walking Test
Patients’ experience
Study participation
Twenty-one of the 57 selected studies reported the number of patients who chose not to engage in rehabilitation interventions. Refusal rates ranged between 2 and 75% (MED = 9.0; IQR = 30), with 5 studies reporting rates higher than 40%. The main reasons cited for refusal were disclosed in only 6 studies. They involved transportation issues [21, 29, 35], a preference for another intervention [23, 31, 37] or requesting their own therapist [29, 31], lack of interest [29, 31] and a desire to minimize hospital appointments in favor of getting back to work, and to a normal lifestyle [45].
Compliance with the study protocol
Adherence to rehabilitation interventions was measured in 19.3% of studies (11 out of 57) and deemed reasonable in each case (see Additional file 2: Table S2 for details). Coordinating therapy sessions with oncologist appointments [21, 39, 45], follow-up calls and positive reinforcement by physical therapists [24, 25, 42, 74], individualization of interventions based on the patient's needs [71], support from spouses or family members [74] and obtaining positive effects from the intervention [55] were identified as factors promoting adherence. Dropout rates were reported in 31 of the 57 included studies and were highly heterogeneous, ranging from 1 to 58% (MED = 10.0; IQR = 12.8). Main reasons stated for not completing the study were undergoing another breast surgery [19, 20, 22, 28, 38, 39, 42, 43, 72, 75], death [19, 20, 22, 24, 31, 35, 37,38,39, 45, 56, 60, 63], cancer recurrence or other medical conditions [19, 20, 22, 24, 31, 32, 35], having to deal with systemic treatment-related AEs [31, 32, 39, 41, 42], moving away [19, 20, 22, 24, 37, 39, 45], lack of interest or time [21, 24, 36] and transportation issues [29, 31]. Two studies also identified lack of support from family and friends [74] and hospital anxiety [19] as barriers to completion.
Adverse events
Only six studies included in this review explicitly discussed the occurrence of AEs. Of these, most studies (5 out of 6) found that the intervention did not affect the patients’ clinical presentation and symptoms. Sagen et al. [39] reported two cases of adhesive capsulitis and one case of supraspinatus tendinopathy. However, the timing of these AEs was not specified, therefore it is unclear whether these are due to the rehabilitation interventions or related to breast cancer treatments. A significant proportion of studies (25 out of 57) also reported that some participants suffered postoperative complications. Among these, lymphedema, seroma, wound dehiscence, and scar contracture were the most frequent. Once again, with little or no description of when these complications occurred, it remains unclear whether these were acute or late effects of breast cancer treatments.
Discussion
This scoping review examined the extent and nature of clinical research on perioperative physical rehabilitation for women with breast cancer who were awaiting or had undergone mastectomy. Our main objective was to identify conservative interventions and relevant clinical outcome measures currently used for this population. As a secondary objective, we aimed to report on barriers and facilitators of participating and completing these interventions. Over half of the eligible studies included mixed breast cancer stages (0-III) populations who underwent various types of breast surgery, axillary procedures, and a series of adjuvant treatments.
Conservative interventions
Rehabilitation programs identified four main modalities: exercise, patient education, manual therapy, and MLD. Multimodal rehabilitation interventions were most frequently reported, all of which included exercise. Rehabilitation interventions consisted primarily of one-on-one sessions initially performed under supervision in hospital settings until discharge. This review also established that rehabilitation interventions were by far the most studied after breast surgery. Only ten interventions were initiated preoperatively, consisting primarily of self-management strategies to be implemented in the postoperative period. Most interventions lasted less than 6 months.
The rehabilitation interventions identified in this scoping review reflect, to some extent, the recommendations provided by cancer care guidelines. However, we noted that the eligible studies had placed less emphasis on aerobic training, primarily providing rehabilitation programs that included exercises targeting upper extremity function. Few identified recommendations concerning rehabilitation strategies to be implemented before surgery, either in the eligible studies or in cancer care guidelines, indicating that further research is needed in this area. In 2017, the World Health Organization (WHO) urged for a coordinated and concerted global action toward improving the accessibility of high-quality rehabilitation services in health systems. Given the systemic effects of cancer and its associated treatments, oncology was designated as a priority area for this initiative [77]. Accordingly, a systematic review was conducted to identify and synthesize rehabilitation-specific recommendations provided by the most recent cancer care guidelines [78]. Of these, the American Cancer Society (ACS)/American Society for Clinical Oncology (ASCO) guideline [79] concluded that there was insufficient evidence to support a specific intervention that would promote optimal postoperative recovery for breast cancer patients. Nevertheless, physical rehabilitation recommendations endorsed by this guideline advised clinicians to encourage their patients to adhere to the ACS’s physical activity recommendations [80], which include moderate to vigorous aerobic exercises and strength training. Returning to normal daily activities as soon as possible after diagnosis and including spouses and family members in usual breast cancer care were also promoted. In turn, to manage breast cancer patients with or at risk for lymphedema, the National Comprehensive Cancer Network Survivorship Guideline [81] recommended a supervised multimodal rehabilitation intervention consisting of progressive resistance training, shoulder ROM exercises, manual lymphatic drainage, education regarding signs and symptoms of postoperative complications and self-care management strategies. This multimodal strategy is also consistent with the recommendations issued from the American College of Sports Medicine guideline [82], which supported the effectiveness of combined moderate-intensity aerobic and progressive resistance training, performed for 8 to 12 weeks, in improving cancer-related health outcomes, including physical functioning, QoL and fatigue. Interestingly, none of these recommendations provided guidance as to what parameters (i.e., frequency, repetitions, sets, etc.) should characterize shoulder ROM exercises. It should also be stressed that these guidelines were primarily derived from studies performed on breast cancer survivors. Therefore, these recommendations may not be fully applicable to breast cancer patients dealing with the acute effects of mastectomy.
Clinical outcome measures
A significant number of outcome measures were used to report the effects of perioperative rehabilitation in breast cancer patients, each of which was measured through a wide range of questionnaires and measurement tools. Objective measures of physical function were the most frequently used and combined with PROMS in over half of the eligible studies. Considering the large spectrum of side effects of breast cancer and its treatments, selecting relevant clinical outcome measures for this population can be challenging. The WHO’s International Classification of Functioning, Disability and Health (ICF) is a common framework that describes health and disability worldwide [83]. As the ICF was considered hardly practical for research and clinical practice, the WHO developed core sets from this classification, which are lists of predetermined outcome measures known to be relevant for specific health conditions [83]. The ICF Core Set for breast cancer [84] covers all the factors that may impact breast cancer patients’ functioning. This model acknowledges that breast cancer patients may experience disabilities not only related to (1) body structures and (2) functions, but also in relation to (3) activities participation and (4) environment interaction [84]. Most studies (39 out of 57) included in this review used outcome measures belonging to at least 2 of the 4 categories of the ICF core sets for breast cancer. Objective measures of physical function were used extensively to account for items pertaining to the first two categories. In contrast, QoL questionnaires were mostly used to report on patients’ ability to carry out activities of daily living and interact with their environment. As QoL is a construct that encompasses many dimensions, the data obtained from these questionnaires may not be as informative. For psychological, social, and environmental factors to be adequately measured, it is advisable to select tools that can provide individual scores for these domains. As an example, the Functional Assessment of Cancer Therapy-Breast Questionnaire (FACT-B) is a questionnaire designed to measure five domains of health-related QoL in breast cancer patients: physical, social, emotional, functional well-being as well as breast cancer-specific concerns [85].
Patients’ experience
This literature review also revealed that a variable proportion of breast cancer patients refused to engage in a rehabilitation intervention despite their eligibility. Studies identified a significant discrepancy in refusal rates. When comparing studies with higher refusal rates to those with lower rates, we noted that these studies had similar characteristics in terms of population, type of interventions, duration, and postoperative complications. However, most studies with higher refusal rates appeared to be conducted partly or entirely in hospital settings. As some wanted to minimize hospital appointments in favor of returning to a normal lifestyle, this information might suggest that transitioning from a supervised inpatient to a home-based intervention or implementing rehabilitation interventions in outpatient clinics or community settings may promote patient engagement. Study withdrawals were mainly attributed to personal or treatment-related factors rather than the intervention itself, which seems to support the appropriateness and safety of rehabilitation interventions for this population. Recognizing the positive impact that support from family and friends had on participants' motivation raises the possibility that breast cancer patients could also benefit from a group intervention, where they could support each other as they go through the same challenges. Tailoring interventions to participants’ needs and circumstances also appears to promote intervention compliance. However, given the small number of studies from which these data were obtained, further work is needed to better document these issues.
Reporting of interventions and outcome measures
We identified several gaps in interventions and harm reporting by relying on the revised CONSORT statement and extensions [76, 86, 87] to guide data extraction. As shown in Additional file 2: Tables S1 and S2, these limitations are such that it remains unclear which parameters should be preferred to promote optimal postoperative recovery in breast cancer patients. Improvements in reporting are needed to ensure patient safety and replicability of interventions in clinical settings. A better description of recruitment and compliance issues arising in this clinical context is also warranted to foster the development of interventions tailored to breast cancer patients’ needs and concerns. As for clinical outcome measures, several studies have used measurement tools and questionnaires without mentioning their validity for the population of interest. To ensure the effects of rehabilitation interventions are accurately measured, future studies should focus on better describing these tools while providing evidence supporting their validity for breast cancer patients.
Limitations
Our scoping review has some limitations. Despite conducting robust systematic searches in multiple relevant databases, we excluded studies not published in English or French (authors’ native language), which may have resulted in relevant studies being missed. However, it has been reported that excluding non-English publications from evidence-syntheses does not lead to bias as it would have a minimal effect on overall conclusions [88, 89]. Some studies were also excluded as they focused on breast cancer survivors. However, some organizations, such as the National Cancer Institute, identify cancer patients as survivors from the day of their diagnosis until the end of their lives [90]. Therefore, studies that did not provide a clear definition of survivorship may have been excluded despite their eligibility. We must also consider that conducting a mixed method scoping review, which would have included qualitative designs, would probably have been better suited to identify barriers and facilitators to study engagement and completion.
Conclusion
This review reports on the variability and wide range of conservative interventions and clinical outcome measures used in physical rehabilitation for breast cancer patients undergoing mastectomy. Exercise, patient education, manual therapy, and MLD were identified as key components characterizing rehabilitation strategies for this population. Although most studies failed to describe interventions’ procedures and characteristics adequately, we were able to determine that most interventions were multimodal, initiated a few days following surgery, and initially performed in supervised hospital settings. More emphasis should be placed on selecting measurement tools and questionnaires that have already been validated for this population. Tailoring interventions to patients’ needs and promoting outpatient rehabilitation interventions appear to be better suited to the particularities of breast cancer care pathways. Ultimately, given the significant heterogeneity characterizing the interventions identified, a better understanding of breast cancer patients’ perioperative care needs and expectations is needed before we can work towards developing rehabilitation resources that can be embedded in our institutions' standards of care.
Availability of data and materials
All data is contained within the manuscript and the additional file.
Abbreviations
- ACS:
-
American Cancer Society
- AES:
-
Adverse events
- ALND:
-
Axillary lymph node dissection
- ASCO:
-
American Society of Clinical Oncology
- BCS:
-
Breast conserving surgery
- CIUSSS-MCQ:
-
Centre intégré universitaire de santé et de services sociaux de la Mauricie-et-du-Centre-du-Québec
- FEV1:
-
Forced expiratory volume in one second
- FVC:
-
Forced vital capacity
- ICF:
-
International Classification of Functioning, Disability and Health
- MLD:
-
Manual lymphatic drainage
- PROMS:
-
Patient-reported outcome measures
- QoL:
-
Quality of life
- RCT:
-
Randomized control trial
- ROM:
-
Range of motion
- WHO:
-
World Health Organization
References
Lagacé F, Ghazawi FM, Le M, Rahme E, Savin E, Zubarev A, et al. Analysis of incidence, mortality trends, and geographic distribution of breast cancer patients in Canada. Breast Cancer Res Treat. 2019;178(3):683–91.
Brenner DR, Weir HK, Demers AA, Ellison LF, Louzado C, Shaw A, et al. Projected estimates of cancer in Canada in 2020. CMAJ. 2020;192(9):E199–205.
Jones C, Lancaster R. Evolution of operative technique for mastectomy. Surg Clin North Am. 2018;98(4):835–44.
Vandal N, Daigle, JM., Hébert-Croteau, N.,Théberge, I., Brisson, J. Breast cancer mortality reduction after initiation of a screening program: consistency of effect estimates obtained using different approaches. In: PQDCS Cdéd (ed) Institut national de santé publique du Québec; 2010.
Perron L, Major D., Hébert-Croteau, N., Brisson, J. Évolution de la détection précoce, l'investigation, le traitement et la survie chez les femmes avec un cancer du sein diagnostiqué entre 1993 et 2003 au Québec. Institut national de santé publique du Québec; 2011.
Santa Mina D, Brahmbhatt P, Lopez C, Baima J, Gillis C, Trachtenberg L, et al. The case for prehabilitation prior to breast cancer treatment. PMR. 2017;9(9S2):S305–16.
McDonald ES, Clark AS, Tchou J, Zhang P, Freedman GM. Clinical diagnosis and management of breast cancer. J Nucl Med. 2016;57(Suppl 1):9S-16S.
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8-30.
Hidding JT, Beurskens CH, van der Wees PJ, van Laarhoven HW, Nijhuis-van der Sanden MW. Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic review. PLoS ONE. 2014;9(5):e96748.
Mirandola D, Miccinesi G, Muraca MG, Belardi S, Giuggioli R, Sgambati E, et al. Longitudinal assessment of the impact of adapted physical activity on upper limb disability and quality of life in breast cancer survivors from an Italian cohort. Support Care Cancer. 2018;26(2):329–32.
De Groef A, Van Kampen M, Dieltjens E, Christiaens MR, Neven P, Geraerts I, et al. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Arch Phys Med Rehabil. 2015;96(6):1140.
Ribeiro IL, Moreira RFC, Ferrari AV, Alburquerque-Sendín F, Camargo PR, Salvini TF. Effectiveness of early rehabilitation on range of motion, muscle strength and arm function after breast cancer surgery: a systematic review of randomized controlled trials. Clin Rehabil. 2019;33(12):1876–86.
Bierman PJ (ed) Self-efficacy for management of symptoms and symptom distress in adults with cancer: an integrative review. Oncology nursing forum; 2019. Oncology Nursing Society.
Chirico A, Lucidi F, Merluzzi T, Alivernini F, De Laurentiis M, Botti G, et al. A meta-analytic review of the relationship of cancer coping self-efficacy with distress and quality of life. Oncotarget. 2017;8(22):36800.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):1–9.
Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. JBI Evid Implement. 2015;13(3):141–6.
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(5):561–4.
Ammitzbøll G, Johansen C, Lanng C, Andersen EW, Kroman N, Zerahn B, et al. Progressive resistance training to prevent arm lymphedema in the first year after breast cancer surgery: results of a randomized controlled trial. Cancer. 2019;125(10):1683–92.
Ammitzbøll G, Kristina Kjær T, Johansen C, Lanng C, Wreford Andersen E, Kroman N, et al. Effect of progressive resistance training on health-related quality of life in the first year after breast cancer surgery—results from a randomized controlled trial. Acta Oncol (Stockholm, Sweden). 2019;58(5):665–72.
Anderson RT, Kimmick GG, McCoy TP, Hopkins J, Levine E, Miller G, et al. A randomized trial of exercise on well-being and function following breast cancer surgery: the RESTORE trial. J Cancer Surv Res Pract. 2012;6(2):172.
Bendz I, Fagevik OM. Evaluation of immediate versus delayed shoulder exercises after breast cancer surgery including lymph node dissection—a randomised controlled trial. Breast (Edinburgh, Scotland). 2002;11(3):241–8.
Beurskens CH, van Uden CJ, Strobbe LJ, Oostendorp RA, Wobbes T. The efficacy of physiotherapy upon shoulder function following axillary dissection in breast cancer, a randomized controlled study. BMC Cancer. 2007;7:166.
Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM. Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Res Treat. 2002;75(1):35–50.
Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM. Physiotherapy after breast cancer surgery: results of a randomised controlled study to minimise lymphoedema. Breast Cancer Res Treat. 2002;75(1):51–64.
Cho Y, Do J, Jung S, Kwon O, Jeon J, Jeon JY. Effects of a physical therapy program combined with manual lymphatic drainage on shoulder function, quality of life, lymphedema incidence, and pain in breast cancer patients with axillary web syndrome following axillary dissection. Support Care Cancer. 2016;24(5):2047–57.
Cinar N, Seckin U, Keskin D, Bodur H, Bozkurt B, Cengiz O. The effectiveness of early rehabilitation in patients with modified radical mastectomy. Cancer Nurs. 2008;31(2):160–5.
de Almeida Rizzi SKL, Haddad CAS, Giron PS, Figueira PVG, Estevão A, Elias S, et al. Early free range-of-motion upper limb exercises after mastectomy and immediate implant-based reconstruction are safe and beneficial: a randomized trial. Ann Surg Oncol. 2020;27(12):4750–9.
De Groef A, Van Kampen M, Vervloesem N, De Geyter S, Christiaens MR, Neven P, et al. Myofascial techniques have no additional beneficial effects to a standard physical therapy programme for upper limb pain after breast cancer surgery: a randomized controlled trial. Clin Rehabil. 2017;31(12):1625–35.
de Rezende LF, Franco RL, de Rezende MF, Beletti PO, Morais SS, Gurgel MS. Two exercise schemes in postoperative breast cancer: comparison of effects on shoulder movement and lymphatic disturbance. Tumori. 2006;92(1):55–61.
Devoogdt N, Geraerts I, Van Kampen M, De Vrieze T, Vos L, Neven P, et al. Manual lymph drainage may not have a preventive effect on the development of breast cancer-related lymphoedema in the long term: a randomised trial. J Physiother. 2018;64(4):245–54.
Feyzioğlu Ö, Dinçer S, Akan A, Algun ZC. Is Xbox 360 Kinect-based virtual reality training as effective as standard physiotherapy in patients undergoing breast cancer surgery? Support Care Cancer. 2020;28(9):4295–303.
Kilbreath S, Refshauge K, Beith J, Lee M. Resistance and stretching shoulder exercises early following axillary surgery for breast cancer. Rehab Oncol. 2006;24(2):9.
Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Lee M, Simpson JM, et al. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2012;133(2):667.
Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol. 2005;44(5):449–57.
Majed M, Neimi CA, Youssef SM, Takey KA, Badr LK. The impact of therapeutic exercises on the quality of life and shoulder range of motion in women after a mastectomy, an RCT. J Cancer Educ. 2020.
Pace do Amaral MT, Freire de Oliveira MM, Ferreira NO, Guimarães RV, Sarian LO, Gurgel MS. Manual therapy associated with upper limb exercises vs exercises alone for shoulder rehabilitation in postoperative breast cancer. Physiother Theory Pract. 2012;28(4):299–306.
Petito EL, Esteves MT, Elias S, Facina G, Nazário AC, Gutiérrez MG. The influence of the initiation of an exercise programme on seroma formation and dehiscence following breast cancer surgery. J Clin Nurs. 2014;23(21–22):3087–94.
Sagen A, Kåresen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol. 2009;48(8):1102–10.
Schultz I, Barholm M, Gröndal S. Delayed shoulder exercises in reducing seroma frequency after modified radical mastectomy: a prospective randomized study. Ann Surg Oncol. 1997;4(4):293–7.
Siedentopf F, Utz-Billing I, Gairing S, Schoenegg W, Kentenich H, Kollak I. Yoga for patients with early breast cancer and its impact on quality of life—a randomized controlled trial. Geburtshilfe Frauenheilkd. 2013;73(4):311–7.
Temur K, Kapucu S. The effectiveness of lymphedema self-management in the prevention of breast cancer-related lymphedema and quality of life: a randomized controlled trial. Eur J Oncol Nurs. 2019;40:22–35.
Teodózio CGC, Marchito LO, Fabro EAN, Macedo FO, de Aguiar SS, Thuler LCS, et al. Shoulder amplitude movement does not influence postoperative wound complications after breast cancer surgery: a randomized clinical trial. Breast Cancer Res Treat. 2020;184(1):97–105.
Testa A, Iannace C, Di Libero L. Strengths of early physical rehabilitation programs in surgical breast cancer patients: results of a randomized controlled study. Eur J Phys Rehabil Med. 2014;50(3):275–84.
Todd J, Scally A, Dodwell D, Horgan K, Topping A. A randomised controlled trial of two programmes of shoulder exercise following axillary node dissection for invasive breast cancer. Physiotherapy. 2008;94(4):265–73.
Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi A, Prieto Merino D, Mayoral del Moral O, Cerezo Téllez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ. 2010;340:b5396.
Wingate L, Croghan I, Natarajan N, Michalek AM, Jordan C. Rehabilitation of the mastectomy patient: a randomized, blind, prospective study. Arch Phys Med Rehabil. 1989;70(1):21–4.
Zhang L, Fan A, Yan J, He Y, Zhang H, Zhang H, et al. Combining manual lymph drainage with physical exercise after modified radical mastectomy effectively prevents upper limb lymphedema. Lymphat Res Biol. 2016;14(2):104–8.
Zhou K, Wang W, An J, Li M, Li J, Li X. Effects of progressive upper limb exercises and muscle relaxation training on upper limb function and health-related quality of life following surgery in women with breast cancer: a clinical randomized controlled trial. Ann Surg Oncol. 2019;26(7):2156–65.
Zimmermann A, Wozniewski M, Szklarska A, Lipowicz A, Szuba A. Efficacy of manual lymphatic drainage in preventing secondary lymphedema after breast cancer surgery. Lymphology. 2012;45(3):103–12.
Fatima T, Shakoor A, Ilyas M, Safdar M, Majeed S. Effectiveness of preoperative stretchings on postoperative shoulder function in patients undergoing mastectomy. JPMA J Pak Med Assoc. 2022;72(4):625–8.
Heiman J, Onerup A, Wessman C, Haglind E, Olofsson BR. Recovery after breast cancer surgery following recommended pre and postoperative physical activity: (PhysSURG-B) randomized clinical trial. Br J Surg. 2021;108(1):32.
Joo OY, Moon SJ, Lee DW, Lew DH, Lee WJ, Song SY. The effect of early arm exercise on drainage volume after total mastectomy and tissue expander insertion in breast cancer patients: a prospective study. Arch Plast Surg. 2021;48(6):583.
Klein I, Kalichman L, Chen N, Susmallian S. A pilot study evaluating the effect of early physical therapy on pain and disabilities after breast cancer surgery: prospective randomized control trail. Breast (Edinburgh, Scotland). 2021;59:286–93.
Odynets T, Briskin Y, Todorova V, Pasichna T, Yefremova A. Effectiveness of yoga intervention enhanced by progressive muscular relaxation on pain in women after breast cancer surgery. Physiother Q. 2021;28(4):25–9.
Paskett ED, Le-Rademacher J, Oliveri JM, Liu H, Seisler DK, Sloan JA, et al. A randomized study to prevent lymphedema in women treated for breast cancer: CALGB 70305 (Alliance). Cancer. 2021;127(2):291–9.
Rizzi S, Haddad CAS, Giron PS, Figueira PVG, Estevão A, Elias S, et al. Exercise protocol with limited shoulder range of motion for 15 or 30 days after conservative surgery for breast cancer with oncoplastic technique: a randomized clinical trial. Am J Clin Oncol. 2021;44(6):283–90.
de Oliveira MM, de Rezende LF, do Amaral MT, Pinto SMP, Morais SS, Gurgel MS. Manual lymphatic drainage versus exercise in the early postoperative period for breast cancer. Physiother Theory Pract. 2014;30(6):384–9.
Na YM, Lee JS, Park JS, Kang SW, Lee HD, Koo JY. Early rehabilitation program in postmastectomy patients: a prospective clinical trial. Yonsei Med J. 1999;40(1):1–8.
Oliveira MMF, Gurgel MSC, Amorim BJ, Ramos CD, Derchain S, Furlan-Santos N, et al. Long term effects of manual lymphatic drainage and active exercises on physical morbidities, lymphoscintigraphy parameters and lymphedema formation in patients operated due to breast cancer: a clinical trial. PLoS ONE. 2018;13(1):e0189176.
Scaffidi M, Vulpiani MC, Vetrano M, Conforti F, Marchetti MR, Bonifacino A, et al. Early rehabilitation reduces the onset of complications in the upper limb following breast cancer surgery. Eur J Phys Rehabil Med. 2012;48(4):601–11.
Huo H, Wang Q, Zhou S, Cui L. The application of personalized rehabilitation exercises in the postoperative rehabilitation of breast cancer patients. Ann Palliat Med. 2021;10(4):4486–92.
Rett MT, Moura DP, de Oliveira FB, Domingos HYB, de Oliveira MMF, Gallo RBS, et al. Physical therapy after breast cancer surgery improves range of motion and pain over time. Fisioterapia e Pesquisa. 2022;29(1):46–52.
Springer BA, Levy E, McGarvey C, Pfalzer LA, Stout NL, Gerber LH, et al. Pre-operative assessment enables early diagnosis and recovery of shoulder function in patients with breast cancer. Breast Cancer Res Treat. 2010;120(1):135–47.
Morimoto T, Tamura A, Ichihara T, Minakawa T, Kuwamura Y, Miki Y, et al. Evaluation of a new rehabilitation program for postoperative patients with breast cancer. Nurs Health Sci. 2003;5(4):275–82.
Paolucci T, Saggino A, Agostini F, Paoloni M, Bernetti A, Mangone M, et al. The influence of rehabilitation on quality of life in breast cancer survivors: a clinical study. Int J Environ Res Public Health. 2021;18(16):5468.
Lu SR, Hong RB, Chou W, Hsiao PC. Role of physiotherapy and patient education in lymphedema control following breast cancer surgery. Ther Clin Risk Manag. 2015;11:319–27.
Kim KH, Yeo SM, Cheong IY, Kim Y, Jeon BJ, Hwang JH. Early rehabilitation after total mastectomy and immediate reconstruction with tissue expander insertion in breast cancer patients: a retrospective case-control study. J Breast Cancer. 2019;22(3):472–83.
Manfuku M, Nishigami T, Mibu A, Yamashita H, Imai R, Tanaka K, et al. Effect of perioperative pain neuroscience education in patients with post-mastectomy persistent pain: a retrospective, propensity score-matched study. Support Care Cancer. 2021;29(9):5351.
Effects of Swiss ball exercise and stretching exercise in chest wall mobility and shoulder range of motion among post-operative breast cancer women. Asian J Pharm Clin Res. 2020;13(4):137–41.
Hsieh CC, Sprod LK, Hydock DS, Carter SD, Hayward R, Schneider CM. Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncol Nurs Forum. 2008;35(6):909–15.
Petito EL, Nazario ACP, Martinelli SE, Facina G, De Gutirrez MGR. Application of a domicile-based exercise program for shoulder rehabilitation after breast cancer surgery. Rev Lat Am Enferm RLAE. 2012;20(1):35–43.
Singh C, De Vera M, Campbell KL. The effect of prospective monitoring and early physiotherapy intervention on arm morbidity following surgery for breast cancer: a pilot study. Physiother Can. 2013;65(2):183–91.
Kilgour RD, Jones DH, Keyserlingk JR. Effectiveness of a self-administered, home-based exercise rehabilitation program for women following a modified radical mastectomy and axillary node dissection: a preliminary study. Breast Cancer Res Treat. 2008;109(2):285–95.
Baima J, Reynolds SG, Edmiston K, Larkin A, Ward BM, O’Connor A. Teaching of independent exercises for prehabilitation in breast cancer. J Cancer Educ. 2017;32(2):252–6.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:874.
Gimigliano F, Negrini S. The World Health Organization rehabilitation 2030: a call for action. Eur J Phys Rehabil Med. 2017;53(2):155–68.
Stout NL, Santa Mina D, Lyons KD, Robb K, Silver JK. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J Clin. 2021;71(2):149–75.
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66(1):43–73.
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):242–74.
Denlinger CS, Sanft T, Baker KS, Broderick G, Demark-Wahnefried W, Friedman DL, et al. Survivorship, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(10):1216–47.
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90.
Stucki G. International Classification of Functioning, Disability, and Health (ICF): a promising framework and classification for rehabilitation medicine. Am J Phys Med Rehabil. 2005;84(10):733–40.
Brach M, Cieza A, Stucki G, Füssl M, Cole A, Ellerin B, et al. ICF Core Sets for breast cancer. J Rehabil Med. 2004;44 Suppl:121–7.
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–86.
Ioannidis JP, Evans SJ, Gøtzsche PC, O’neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–8.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):1–8.
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138.
Nussbaumer-Streit B, Klerings I, Dobrescu A, Persad E, Stevens A, Garritty C, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2020;118:42.
Institute NC. NCI dictionary of cancer terms. United States Governments
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
JM participated to the study conception and selection, extracted and analyzed the data and wrote the first draft of the manuscript; CD collected data; NL contributed to the study selection and extracted data; AD contributed to the study conception; AAM contributed to study conception, revised the manuscript and supervised the work; MD contributed to the study conception, checked the extracted data and supervised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. MEDLINE search strategy
Additional file 2
. Summary of included studies and description of rehabilitation the interventions
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mathieu, J., Daneau, C., Lemeunier, N. et al. Conservative interventions and clinical outcome measures used in the perioperative rehabilitation of breast cancer patients undergoing mastectomy: a scoping review. BMC Women's Health 22, 343 (2022). https://doi.org/10.1186/s12905-022-01927-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-01927-3