Abstract
Background
Chlamydia trachomatis infection and pelvic inflammatory disease (PID) are well-known risk factors for female infertility. But there is limited evidence from China. This study aimed to further explore the associations between previous/current chlamydial infection, PID, and infertility in China.
Methods
We performed a 1:2 matched case–control study with two control groups: pregnant controls and non-pregnant controls in China in 2019. Women diagnosed with infertility were selected as cases (n = 255). Controls were selected based on the following criteria: Pregnant women who were documented in the selected hospitals were chosen as Pregnant controls (n = 510), and people who sought health care in Obstetric/Gynecologic clinics, Family Planning clinics, Dermatology and STD Department or Urological department were selected as Non-pregnant controls (n = 510). Infertility induced by male factors and people who used antibiotics in the vagina within two weeks were excluded. The first-stream specimen of urine samples was tested for chlamydia by nucleic acid amplification testing (NAAT). Conditional logistic regression was used to estimate the association.
Results
The prevalence of previous chlamydial infection and PID were significantly higher in cases (2.4%, 17.3%) than in controls (Non-pregnancy: 0.4%, 3.0%; Pregnancy: 0.4%, 9.0%). The current chlamydial infection rates were 5.9%, 7.3%, and 7.1% in infertile, pregnant, and non-pregnant women, respectively. After adjusting for confounders, PID largely elevated the risk of infertility (using non-pregnant controls: adjusted OR = 2.57, 95% CI 1.51, 4.39; using pregnant controls: adjusted OR = 6.83, 95% CI 3.47, 13.43). And the positive association between PID and tubal infertility was more obvious for both groups. For current chlamydial infection, none of the odds ratios were significant at the 0.05 level, while small sample size limited the evaluation of an association between prior chlamydial infection with infertility.
Conclusions
Previous PID was indicated to largely increase the risk of infertility, especially tubal infertility. And there should be continuing emphasis on highly sensitive and specific biomarker for prior chlamydial infection.
Similar content being viewed by others
Background
Infertility refers to the failure to achieve a clinical pregnancy after over one year of regular unprotected sexual intercourse [1]. Infertility is a highly prevalent global condition and the incidence is on the rise, which is estimated to affect around 9% of reproductive-aged couples worldwide [2]. In China, the overall prevalence of infertility has reached 15.5%, while among women actively trying to conceive, the prevalence is as high as 25% [3]. Quite a few studies have revealed a negative impact of infertility on endometrial, myometrial, cervical, and placental alterations that underlie the poor obstetric outcomes [4]. It not only creates a considerable cost burden but also introduces tensions to the family. Further insight into the causes is critical to help alleviate the burden.
Chlamydia trachomatis is one of the most prevalent sexually transmitted microorganisms. Ascending from the lower genital tract, chlamydial infection can lead to serious reproductive consequences including infertility [5]. It was estimated that the proportion of tubal infertility caused by chlamydia had reached 45% [5]. Furthermore, this sexually transmitted microorganism is an important cause of pelvic inflammatory disease (PID), and PID was also indicated to increase the risk of infertility, then PID represents the link between chlamydia infection and infertility [6,7,8]. However, despite numerous studies identifying chlamydial infertility, the true host and pathogen determinants underlying infertility remain unknown due to the lack of appropriate experimental models and suitable diagnostic tools [5, 9], and the current evidence base was weak for several reasons. First, the poor performance of serological tests that were commonly used in those studies [5]. Second, pregnant women were commonly selected as control participants, of whom are different from the source population and generally exaggerate the association [10].
China provided a great opportunity to further explore the association between chlamydial infection, PID and infertility for the following reasons. First, China has a considerable disease burden of chlamydial infection. According to the National Notifiable Infectious Disease Reporting System, the reported annual incidence rate in females was 84.55 per 100,000 population in 2019, which was highly underestimated due to the insufficient chlamydia screening/testing and poor detection methods [11]. Second, the implementation of the universal two-child policy in recent years encouraged many people to seek care for infertility, which increased our access to infertile women.
In this case–control study, we aimed to investigate whether previous/current chlamydial infection and previous PID would influence the risk of infertility in the Chinese population.
Methods
Study population
This was a 1:2 matched case–control study aiming to explore the effects of chlamydial infection and PID on infertility. Women aged between 18 to 44 years were considered for the study if they sought for health care between Jan 1, 2019, and Oct 30, 2019, at outpatient settings, including clinics or auxiliary reproductive center, in five cities (Guangzhou, Zhanjiang, Shenzhen, Dongguan, Yunfu) of Guangdong Province, China [12].
Infertility was defined as failure to conceive after 12 months or more of regular unprotected sexual intercourse [1]. All cases involved in our study underwent a diagnostic workup by professionals, and if the patients self-reported infertility, they would receive laparoscopy and hysterosalpinography for further verification. And according to the American Society for Reproductive Medicine, we further classified infertility into several subtypes, including tubal disorders, endocrine disturbance, cervical/uterine/peritoneal factors, immune factors, sex factors, and others or unknown [13]. The participants were excluded if they met one of the following conditions: (1) infertility induced by male factors, and (2) who used antibiotics in the vagina within two weeks.
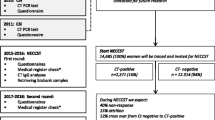
We performed a 1:2 matched case–control study with two control groups: pregnant controls and non-pregnant controls. For each infertility case that was ascertained, two control women were matched by age (± 3 years). For pregnant controls, we included pregnant women who were documented in the selected hospitals of the study cities. As non-pregnant controls, we selected those who sought health care other than infertility in Obstetric/Gynecologic clinics, Family Planning clinics, Dermatology & STD Department or Urological department for the first time in the past one year, and reported ever having sexual intercourse and willing to be tested for chlamydia. Indications for exclusion were the same with that of cases. Finally, we included 255 infertile women, 510 pregnant women (control 1), and 510 non-pregnant women (control 2) in this study. Information about baseline characteristics including sociodemographic characteristics, reproductive history, smoking and alcohol drinking, previous history of diseases, and sexually transmitted infection (STI) were collected through online questionnaires (Fig. 1).
The study was approved by the Institutional Review Board of Dermatology Hospital of Southern Medical University. All participants have given written informed consent.
Sample collection and nucleic acid amplification testing
The first-stream specimen of urine samples was collected from all participants. At least one-hour interval before the last urination was needed. Self-cleaning of the urination site was not allowed. After collection, transferred it into cobas®PCR MEDIA tubes and mixed upside down five times. Then stored the sample under 2–8 °C. Cobas® 4800 (Cobas® 48000 CT/NG Amplification/Detection Kit, Shanghai, China) was used to process urine specimens for the diagnosis of chlamydia trachomatis.
According to the Health Industry Standard of the People's Republic of China (WS/T 513-2016), women with positive NAAT result but without any related symptoms such as odynuria, frequent and/or urgent micturition, hypogastralgia, lumbago, abnormal vaginal bleeding, and/or discharge, would be diagnosed as asymptomatic chlamydia infection. And those with positive NAAT result and related symptoms would be regarded as symptomatic chlamydia infection.
Sample size calculation
In this matched 1:2 case–control study, we supposed the prevalence of chlamydial infection among women to be 4.7% [14], the odd ratio of chlamydial infertility to be 1.91 [15], α = 0.05, β = 0.2. Finally, a sample of 239 infertile cases was obtained, and controls were supposed to be 478 per group.
For PID, about 4.4% of sexually active women received a diagnosis of PID in their lifetime [16], and self-reported PID was associated with a fourfold higher risk of infertility [6]. Then, a sample of 36 infertile cases with matched 72 non-infertile controls can achieve 81% power under α = 0.05.
Overall, at least 239 infertile cases with matched 478 non-infertile controls were needed.
Statistical analysis
We used the chi-square test to evaluate the baseline characteristics between non-pregnant/pregnant women and infertile women. Associations between chlamydial infection, including NAAT verified infection and self-reported previous infection, and PID with infertility in women were studied by conditional logistic regression, calculating odds ratios (ORs) with their corresponding 95% confidence intervals (CIs), adjusting for maternal age, BMI, monthly income, chronic disease, and other genital tract infection, which were suggested to be potential risk factors of infertility by published study [8]. Besides, previous chlamydial infection was added to adjust for the association between PID and infertility.
We conducted several sensitivity analyses. Firstly, we compared different effects on primary infertility and secondary infertility. The former refers to those women who have not been pregnant previously, while the latter is denoted for the inability to repeat conception after a prior pregnancy [2]. Then, considering that some of the infertility subtypes were probably not to be associated with chlamydial infection or PID, we kept infertility cases induced by tubal disorders only and excluded those related to sex factors and/or cervical/uterine/vaginal factors.
Data analyses were performed using Stata 14 software (StataCorp LLC, Texas). Statistical significance was considered at a two-sided P value < 0.05.
Results
Social demographic characteristics
Our study involved 1275 participants, including 510 non-pregnant women, 510 pregnant women, and 255 infertile women, with an average age (\({\overline{\text{x}}}\) ± s) of 30.0 ± 4.5, 29.8 ± 4.4 and 29.8 ± 4.4 years, respectively. We compared baseline characteristics and previous history of diseases between non-pregnant/pregnant women and infertile women (Table 1). Most participants only received high school education or lower and earned less than $1500 (about 10,000 RMB) per month. And compared with control groups, more infertile women earned over $1500 per month for (P < 0.05). The (pregestational) BMI of infertile women was higher than that of pregnant women. For non-pregnant women, the age of sexual debut was lower than that of infertile women (P < 0.05). Pregnant women were more likely to have chronic diseases but less likely to experience PID and other genital tract infections in the past.
As mentioned above, we further classified infertility into several subtypes, and Fig. 2 showed that tubal disease was an important cause of infertility, which accounted for 34% of our cases. And endocrine disturbance (20%) was also worth attention.
Chlamydial infection
The prevalence of self-reported previous chlamydial infection was significantly higher in infertile women (2.4%) than in controls (Non-pregnancy: 0.4%; Pregnancy: 0.4%). And the current infection rates tested by NAAT were 5.9%, 7.1%, and 7.3% in infertile, non-pregnant and pregnant women, respectively. In this study, the percentage of asymptomatic infection was lower among control groups (non-pregnancy: 26.5%, pregnancy: 22.7%) than cases (46.2%).
The association between chlamydial infection, previous PID, and infertility
Our study found that previous PID diagnoses were associated with an increased risk of infertility using non-pregnant controls (adjusted OR = 2.57, 95% CI 1.51, 4.39) and pregnant controls (adjusted OR = 6.83, 95% CI 3.47, 13.43). For current chlamydial infection, none of the odds ratios were significant at the 0.05 level (Table 2). Due to the low prevalence of self-reported chlamydia infection, it was not a valid variable for this analysis, so the result was not shown.
Sensitivity analysis
We further evaluated the effects of PID on different subtypes of infertility. Firstly, we compared primary infertility (n = 168) and secondary infertility (n = 87). PID history was positively associated with primary infertility using non-pregnant controls (adjusted OR = 4.41, 95% CI 1.21, 16.12) and using pregnant controls (adjusted OR = 3.78, 95% CI 1.15, 12.41) (Table 3).
Further, we kept infertility cases caused by tubal disorders (n = 86) only, and the positive association between PID and infertility was more obvious for both groups (see Additional file 1: Table S1). Previous PID was associated with an increased tubal infertility risk of 7.98-fold (95% CI 2.76, 23.06) for the non-pregnancy control group and 16.17-fold (95% CI 4.59, 56.97) for the pregnancy control group. Finally, we dropped infertility cases caused by sex factors and/or cervical/uterine/vaginal factors (n = 48), which may be less attributed to maternal infection and PID. Additional file 1: Table S2 showed that the effect size was also elevated to some extent (non-pregnant group: adjusted OR = 3.09, 95% CI 1.66, 5.76; pregnant group: adjusted OR = 7.29, 95% CI 3.37, 15.77).
Discussion
In our evaluation of the association between chlamydial infection, PID and infertility, we found that previous PID was indicated to largely elevate the risk of infertility, especially tubal infertility. The prevalence of infertility among women with self-reported previous chlamydia infection was six times the prevalence among women with negative results, although our sample size limited an evaluation of an association of infertility with it. For current chlamydial infection, none of the odds ratios were significant at the 0.05 level. This study extended the existing literature by using two groups of controls and using NAAT methods to improve the testing accuracy.
Previous PID was indicated to elevate the risk of infertility in this study, and this finding aligned with the existing literature. A national health and nutrition examination survey conducted in the United States found that self-reported PID was associated with a fourfold higher risk of infertility among young women (18–29 years old) [6]. Another study conducted in China using a case–control study design also drawn similar conclusions [7]. Besides, the effect was stronger in pregnant women's control settings than non-pregnant control setting in our study, which may be due to the confounding of infertile cases among non-pregnant women who were unaware of their infertile facts. PID is common and approximately 4.4% sexually active women were reported to have PID [17], and its long-term harmful effects on reproductive disability put an urgent need of public health prevention measures. On the one hand, efforts can be made on identifying noninvasive PID biomarkers considering the current unspecific invasive diagnostic methods [18]. On the other hand, Chlamydia trachomatis is a potentially important cause of PID, and implementing related screening programs would be the most important public health measure for the prevention of PID [18].
The number of self-reported prior chlamydial infection was limited in this study, which may be due to recall bias and low detection rate in China, therefore we did not conduct the association analysis between infertility with it. When indicating prior chlamydial infections, currently available methods include self-reports and chlamydial serologic assays with poor sensitivity, leading to incomplete understanding of chlamydial PID and infertility, so it is a necessity to find an appropriate marker. It is suggested that Pgp3 antibody may better satisfy this need [16]. Our team used an ultrasensitive and high-throughput luciferase immunosorbent assay, with good specificity and sensitivity, for detection of anti-Pgp3 antibodies among reproductive-aged women, including some infertile women (unpublished). Future studies will continue for Pgp3, chlamydia, PID and infertility. From early teenagers before infection until after being a mother, a wider population level follow-up cohort study would be the most robust way to understand its natural history, although it seemed not to be feasible [19].
The association between PID and infertility should be taken in the context of the study design and other limitations beyond those already mentioned. It was unavoidable to bring recall bias when applying a case–control design. Previous PID cases, as exposures of interest, were recalled based on their past diagnosis and may be biased due to the respondents’ distorted or incomplete memory, but we combined objective records from the Hospital Information System and doctors helped the participants to finish the questionnaire. This may reduce recall bias to some extent. Then, Berkson's bias cannot be ignored when analyzing the relationship between current chlamydial infection and infertility. Recruited from hospitals, infertile women involved in this study have possibly experienced a series of examinations including chlamydia testing and further received treatment, which may explain the relatively low rate of NAAT (+) and the biased effects observed. But it was noteworthy that infertility was a long-term consequence, and it was obscure whether infection occurred before infertility among women with positive NAAT.
Conclusions
In summary, previous PID was suggested to be associated with an elevated risk of infertility, especially tubal infertility. And for further assessment of the burden of chlamydia infertility, finding sensitive biomarkers that might predict prior chlamydial infection is urgently needed, because for most women present for infertility, inciting infection would no longer be detected by NAAT or other tests for active urogenital infection.
Availability of data and materials
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PID:
-
Pelvic inflammatory disease
- STI:
-
Sexually transmitted infection
- NAAT:
-
Nucleic acid amplification testing
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
References
Gurunath S, Pandian Z, Anderson RA, Bhattacharya S. Defining infertility—a systematic review of prevalence studies. Hum Reprod Update. 2011;17(5):575–88.
Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–26.
Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, Cao Y, Chen X, Zhu Y, Xu S, et al. Epidemiology of infertility in China: a population-based study. BJOG. 2018;125(4):432–41.
Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, Petraglia F. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22(1):104–15.
Menon S, Timms P, Allan JA, Alexander K, Rombauts L, Horner P, Keltz M, Hocking J, Huston WM. Human and pathogen factors associated with chlamydia trachomatis-related infertility in women. Clin Microbiol Rev. 2015;28(4):969–85.
Anyalechi GE, Hong J, Kreisel K, Torrone E, Boulet S, Gorwitz R, Kirkcaldy RD, Bernstein K. Self-reported infertility and associated pelvic inflammatory disease among women of reproductive age-national health and nutrition examination survey, United States, 2013–2016. Sex Transm Dis. 2019;46(7):446–51.
Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo, Brazil). 2018;73:e364.
Group TECW. Physiopathological determinants of human infertility. Hum Reprod Update. 2002;8(5):435–47.
Kessler M, Hoffmann K, Fritsche K, Brinkmann V, Mollenkopf HJ, Thieck O, Teixeira da Costa AR, Braicu EI, Sehouli J, Mangler M, et al. Chronic Chlamydia infection in human organoids increases stemness and promotes age-dependent CpG methylation. Nat Commun. 2019;10(1):1194.
Wallace LA, Scoular A, Hart G, Reid M, Wilson P, Goldberg DJ. What is the excess risk of infertility in women after genital chlamydia infection? A systematic review of the evidence. Sex Transm Infect. 2008;84(3):171–5.
Yue Xiaoli GX, Jing L, Jiahui Z. Epidemiologic features of genital Chlamydia trachomatis infection at national sexually transmitted disease surveillance sites in China, 2015–2019. Chin J Dermatol. 2020;53(8):596–601.
Li C, Tang W, Ho HC, Ong JJ, Zheng X, Sun X, Li X, Liu L, Wang Y, Zhao P, et al. Prevalence of chlamydia trachomatis among pregnant women, gynecology clinic attendees, and subfertile women in guangdong, china: a cross-sectional survey. Open Forum Infect Dis. 2021;8(6):ofab06.
Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2015;103(6):e44-50.
Chen H, Luo L, Wen Y, He B, Ling H, Shui J, He P, Hou X, Tang S, Li Z. Chlamydia trachomatis and human papillomavirus infection in women from southern Hunan province in China: a large observational study. Front Microbiol. 2020;11:827.
Tang W, Mao J, Li KT, Walker JS, Chou R, Fu R, Chen W, Darville T, Klausner J, Tucker JD. Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: a global systematic review and meta-analysis. Sex Transm Infect. 2020;96(5):322–9.
Anyalechi GE, Hong J, Danavall DC, Martin DL, Gwyn SE, Horner PJ, Raphael BH, Kirkcaldy RD, Kersh EN, Bernstein KT. High plasmid gene protein 3 (Pgp3) Chlamydia trachomatis seropositivity, pelvic inflammatory disease, and infertility among women, national health and nutrition examination survey, United States, 2013–2016. Clin Infect Dis. 2021;73(8):1507–16.
Hillier SL, Bernstein KT, Aral S. A review of the challenges and complexities in the diagnosis, etiology, epidemiology, and pathogenesis of pelvic inflammatory disease. J Infect Dis. 2021;224(12 Suppl 2):S23-s28.
Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372(21):2039–48.
Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, Marrazzo JM, Sonder GJB, Schwebke JR, Hoornenborg E, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17(8):e235–79.
Acknowledgements
Not Applicable.
Funding
This work was supported by Guangdong Medical Science and Technology Research Fund (Grant No. C2019122). Funding sources had no role in study design, analysis and interpretation of data, writing of the report, or the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design and have read and approved the manuscript. LL, CL and WT were responsible for conceptualization, investigation, methodology and formal analysis. LL drafted the original paper. CW, BY and HZ were responsible for methodology and investigation. XS and JL were responsible for acquisition of data and data curation. CW was responsible for funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of Dermatology Hospital of Southern Medical University. All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Sensitivity analysis of the association between chlamydial infection, previous PID and infertility subtypes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, L., Li, C., Sun, X. et al. Chlamydia infection, PID, and infertility: further evidence from a case–control study in China. BMC Women's Health 22, 294 (2022). https://doi.org/10.1186/s12905-022-01874-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-022-01874-z