Abstract
Background
Malignant endometriosis in an episiotomy scar is rare; only seven cases have been reported previously. Here, we compare two cases of benign endometriosis and clear cell carcinoma.
Case presentation
The first case was a 54-year-old woman who presented with a large perineal lesion in her episiotomy scar with high 18F-fluorodeoxyglucose uptake. This location had a history of endometriosis many years ago. She underwent radical excision of the mass and bilateral inguinal lymph node dissection. Histological and immunohistochemical analysis confirmed the presence of clear cell carcinoma arising from endometriosis. Assisted radiotherapy was performed after surgery due to a positive lymph node. No recurrence was detected over a 1-year follow-up period. The second case deals with a 3 × 2 cm mass in the episiotomy scar of a 33-year-old woman. Part of the anal sphincter was resected because of the close proximity of the lesion. Because the disease lay very close to the anus, she received anal sphincter reconstruction combined with mass excision. Pathology result showed typical endometrial glands and interstitial tissues.
Conclusions
Deleterious change only happens in patients experiencing perineal endometriosis. Complete excision is crucial for this form of disease; sometimes impairment of the anal sphincter is also necessary. Patients with malignancy required a combination of treatments in order to improve their prognosis.
Similar content being viewed by others
Background
Endometriosis is a common disease in women and is characterized by the presence of ectopic functional endometrial tissue as an inflammatory condition [1]. Endometriosis can occur at any location, but the incidence of perineal endometriosis is rare and is usually associated with previous episiotomy or other forms of vulvar surgeries [2]. Its mechanism may be the transplantation seeding of endometriosis [3]. Sometimes it is difficult to diagnose such cases without careful case history and clinical findings. The malignant transformation of endometriosis was first described by Sampson in 1925 [4], but cases of malignancy arising from scar endometriosis are extremely rare. The literature relating to this disease consists of only four cases of clear cell carcinoma and one patient with serous papillary cystadenocarcinoma [5,6,7,8]. Here, we report two cases of endometriosis with previous episiotomy scars, one was benign, the other was clear cell carcinoma. In this article, we summarize the clinical features and pathological findings for these patients.
Case presentation
Case 1
A 54-year-old female patient presented with a perineal lump which had gradually enlarged over a period of 4 years. Her obstetric history included a vaginal delivery with a left episiotomy 30 years ago. After that, she remained well for 3 years before she felt a small mass in the incision site. She then underwent resection of the mass and was diagnosed with endometriosis. However, this disease relapsed after a few months, and the patient suffered, and underwent the same operation again. After the second operation, she still felt incisional pain during menstruation. Danazol had been prescribed for 1 year, which relieved the pain. Following drug therapy, 18 years passed and the patient underwent radical mastectomy and chemotherapy due to breast carcinoma in 2012.
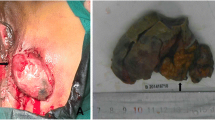
Four years ago, she found a slow-growing perineal mass in the same region. She went to our hospital and physical examination revealed a soft mass, 6 × 5 cm in size, which was closely related to the anterior episiotomy scar (Fig. 1a). Color Doppler ultrasound revealed a well-defined mass in the left perineal area which measured 9.5 × 4.4 × 5.8 cm. Color Doppler flow imaging (CDFI) showed blood flow signals (Fig. 1b, c). Tumor markers CA125, AFP, CA-19-9, CEA, HE4 and SCCA were all normal. We performed enhanced pelvic computed tomography (CT) and identified an irregular soft tissue image between the left region of the anus and vagina (Fig. 1d). We also detected an enlarged lymph node in the left inguinal region. Both of these regions showed enhancement in the scan. Lymph node aspiration biopsy suggested epithelial neoplasm. Combined positron emission tomography and computed tomography (PET/CT) further showed focal increased 18F-fluorodeoxyglucose uptake in both of the tumor and the inguinal lymph node (Fig. 1e, f).
a Clinical appearance of the large mass. b, c Ultrasound and CDFI image of the perineal tumor, measuring 9.5 × 4.4 × 5.8 cm. d CT of recurrence at left perineum incision. e, f Appearance of the mass at PET/CT-scan, it showed an increased uptake of 18F-fluorodeoxyglucose. g, h Intact and dissected surgical specimen
We performed a radical excision of the tumor and bilateral inguinal lymph node dissection. During the surgery, we found the tumor was firm and tan or red-tan in color. The tumor had extended into the deep part of her pelvis, and the interface between it and the surrounding tissue was not well demarcated. Pathological analysis indicated clear cell carcinoma of the perineum incision, possibly arising from an endometrial implant (Fig. 2a-c). A transitional dysplastic zone between the endometriotic focus and the clear cell carcinoma was identified (Fig. 2d). Each side of the superficial inguinal region had one positive lymph node. Immunohistochemically, the tumor cells were positive for CK, P16, HNF1β and AMACR. The patient was discharged after 30 days of hospitalization without any complication. This was followed by 1 month of radiation therapy.
Microscopic images: a Clear cell carcinoma: marked papillary growth, abundance of cellulars and haemorrhage, necrosis, heteromorphism are manifest. b Typical hobnail cells could be found in our malignant case. c endometrial glands are noted in dense stroma. d The symbol of clear cell carcinoma arising from the endometriosis: Transitional zone. Confused structure of cells and disorder of nucleus. Immunohistochemical results demonstrated positve expression of CK and HNF1β (e, f), negenative expression of P53 and progesteron receptor (g, h)
Case 2
A 33-year-old Asian woman presented with a painful mass in her perineal incision, which she had noticed several months before. There was no particular medical history except that she had a vaginal delivery with episiotomy 12 years previously. Subsequently, she felt occasional incisional pain. Four years ago, she had another natural labor and the pain became worse. Over the last few months, the patient could feel a subcutaneous mass in the perineal incision. An ultrasound scan showed a hypoechoic nodule measuring 3.1 × 1.6 × 1.2 cm (Fig. 3a); its margin was clear and there was no enhanced spot echo. A minor blood flow signal was present on CDFI. Following the administration of intravenous contrast material, pelvic magnetic resonance imaging (MRI) revealed a sharply defined subcutaneous solid mass which was strictly adherent to the left side of the anus. (Fig. 3b). On palpation, a soft tumor was identified which lay in the midpoint of the scar (Fig. 3c). Serum levels of CA125, CEA and SCCA were normal, although CA199 was marginally increased at 41.5 Ku/l. At the end, the patient received a complete excision of the perineal mass that was carried out uneventfully. The focus appeared as a hard mass with an irregular shape and ill-defined margins. Part of the anal sphincter was resected because the lesion lay very close. The surgical specimen contained several brown capsulated tissues (Fig. 3d). Areas of necrosis, hemorrhage and cyst formation could be seen on sectioning. Histological examination revealed endometrial glands and a typical interstitium (Fig. 3e, f).
a Sonographic image showed a 3.1 × 1.6 cm hypoechoic lesion. b MRI image showed a subcutaneous solid mass near the left side of the anus on T2WI. c Dark nodule in left episiotomy scar. d Dissected surgical specimen showed a mass with old hemorrhage. e, f Endometrial glands with typical stroma were showed in histological examination
Discussion and conclusions
Functioning endometrial tissue located outside of the uterine cavity is defined as endometriosis [9]. Cutaneous endometriosis is rare and mostly occurs in the abdominal wall, usually developing at the site of a caesarian scar. Perineal and vulvar lesions are more rare; the iatrogenic transplantation of endometrial cells via an episiotomy scar appears to be the mechanism involved [10]. Both of our cases support this hypothesis. These lesions appear as a blue-black nodule under the surgical scar, accompanied by cyclic pain and enlargement during menstruation. Three criteria for diagnosing malignant endometriosis were first proposed by Sampson in 1925: menstrual irregularity, a continuously enlarging mass and increased pain [4].
Tumor markers are not very sensitive for extraovarian lesions, even in malignant cases. Previously, Cuisenier reported that the levels of CA125 are normal in almost half of patients with extraovarian lesions but are normal in only 15.38% of cases with ovarian endometriosis [11]. Both of our patients had normal levels of CA 125. Ultrasound and MRI can help us to identify the exact anatomical position, and in our experience, MRI is highly sensitive and offers excellent differentiation of endometriosis from neighboring tissue; this is important for diagnosis and operative management. The differential diagnosis should include anal cancer, abscesses, fistula, atheroma and hidradenitis.
Malignant degeneration of cutaneous endometriosis is extremely rare, representing 0.3–1% of surgical scars [5]; and its origin is still unclear, although we know that some specific events occur in this process. On the one hand, the mutation of tumor suppressor genes, such as PTEN and P53 may be related to the development of endometriosis associated cancer [12]. The pathological results of our malignant patient were negative for P53, but positive for HNF1β and AMACR. On the other hand, hormones are also known to play a role. It has been confirmed that high levels of estrogen are consistent with the progression of endometrioid cancer and clear cell carcinoma. In addition, inflammatory reactions and cytokines such as IL-1, which can cause angiogenesis, proliferation and the inhibition of apoptosis, can also contribute to the development of this disease [13].
Benign and malignant perineal endometriosis are difficult to distinguish by symptoms or signs [14]. However, we identified a key point in that the malignant diseases are recurrent. The malignant patient in our report, and those in the existing literature, had undergone a perineal mass excision previously. This means that the initial case of this disease is always benign, but the transition of malignancy occurs after several years if the tumor relapses. Because all of the known malignant patients described in the literature, our present patient included, had undergone a resection previously, we suspect that the tumor had not been totally removed during the first surgery. After a long period of stimulation by local inflammation and hormones, these tumors ultimately became malignant. Thus, resection of the whole endometrial mass is crucially important during initial treatment, especially for patients with anal sphincter involvement. The diagnostic accuracy of anal sphincter involvement could be improved by the use of anal endosonography [3, 15]. Previous authors have recommended wide excision of endometrioma with primary sphincteroplasty for these patients [16], and that this is particularly important if the tumor boundary is unclear. Thus, a safe resection margin of more than 0.5 cm of surrounding healthy tissue is necessary in order to avoid relapse or malignancy. To achieve wide excision in cases of perineal endometrioma with anal sphincter invasion, partial removal of external anal sphincter is necessary [16, 17].
Some clinicians have suggested the use of oral drugs to manipulate hormone levels [18,19,20]. However, assisted drug therapy has not been proven to be effective in controlling or postponing the recurrence of perineal endometriosis [21]. For our patient, the use of danazol achieved symptomatic relief but did not prevent recurrence or malignant change. Because of the low incidence of perineal endometriosis, the ideal treatment is still unknown. Table 1 shows previously reported cases of malignant transformation of episiotomy scar endometriosis. In recent literature, a total of four patients with clear cell carcinoma and one patient with serous papillary cystadenocarcinoma have been reported [5,6,7,8, 22]. All of these patients had a history of benign perineal endometriosis. Methods of treatment include radical excision, radiotherapy and chemotherapy. Most of these patients received combined therapies. For our patient, we performed radical surgery and post-operative radiation. Although the results of long-term follow-up remain unknown, a 1 year period of follow up shows no recrudescence or metastasis.
Perineal endometriosis is rare but should be suspected if there is a history of episiotomy and cyclic pain. Ultrasonography, magnetic resonance imaging and PET/CT can be used for diagnosis. Complete resection of the nidus is key to treating this disease because malignancy only happens in recurrent patients. To achieve this, we should consider two key points: (1) adequate and wide excision is the principle of management to prevent recurrence and future malignancy; and (2) we must be very careful not to rupture tumors during surgery as this can cause remnants to remain and subsequent re-implantation to occur. The effect of assisted drug therapy after surgery is not very clear. Adjunctive chemotherapy and radiotherapy are recommended as the prognosis may be improved in malignant patients. Finally, it is very important to follow-up these patients with care.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AFP:
-
Alpha fetoprotein
- AMACR:
-
Alpha-methylacyl-CoA racemase
- CA125:
-
Cancer antigen 125
- CA199:
-
Carbohydrate antigen 19–9
- CEA:
-
Carcinoembryonic antigen
- CK:
-
Cytokeratin
- CT:
-
Computer Tomography
- HE4:
-
Human Epididymis Protein 4
- HNF1β:
-
Hepatocyte nuclear factor 1β
- IL-1:
-
Interleukin-1
- P16:
-
Multiple tumour suppressor 1
- P53:
-
Cellular tumour antigen
- PET/CT:
-
Positron Emission Tomography/Computed Tomography
- PTEN:
-
Phosphatase and tensin homologue deleted on chromosome 10
- SCCA:
-
Squamous cell carcinoma-associated antigen
References
Johnson NP, Hummelshoj L. Consensus on current management of endometriosis. Hum Reprod. 2013;28(6):1552–68.
Zhu L, Lang J, Wang H, Liu Z, Sun D, Leng J, et al. Presentation and management of perineal endometriosis. Int J Gynaecol Obstet. 2009;105(3):230–2.
Watanabe M, Kamiyama G, Yamazaki K, Hiratsuka K, Takata M, Tsunoda A, et al. Anal endosonography in the diagnosis and management of perianal endometriosis: report of a case. Surg Today. 2003;33(8):630–2.
Sampson JA. Endometrial carcinoma of ovary arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–12.
Chene G, Darcha C, Dechelotte P, Mage G, Canis M. Malignant degeneration of perineal endometriosis in episiotomy scar, case report and review of the literature. Int J Gynecol Cancer. 2007;17(3):709–14.
Hitti IF, Glasberg SS, Lubicz S. Clear cell carcinoma arising in extraovarian endometriosis: report of three cases and review of the literature. Gynecol Oncol. 1990;39(3):314–20.
Todd RW, Kehoe S, Gearty J. A case of clear cell carcinoma arising in extragonadal endometriosis. Int J Gynecol Cancer. 2000;10(2):170–2.
Kwon YS, Nam JH, Choi G. Clear cell adenocarcinoma arising in endometriosis of a previous episiotomy site. Obstet Gynecol. 2008;112(2 Pt 2):475–7.
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–99.
Gunes M, Kayikcioglu F, Ozturkoglu E, Haberal A. Incisional endometriosis after cesarean section, episiotomy and other gynecologic procedures. J Obstet Gynaecol Res. 2005;31(5):471–5.
Patrelli TS, Berretta R, Gizzo S, Pezzuto A, Franchi L, Lukanovic A, et al. CA 125 serum values in surgically treated endometriosis patients and its relationships with anatomic sites of endometriosis and pregnancy rate. Fertil Steril. 2011;95(1):393–6.
Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol. 2012;124(1):164–9.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45.
Li J, Shi Y, Zhou C, Lin J. Diagnosis and treatment of perineal endometriosis: review of 17 cases. Arch Gynecol Obstet. 2015;292(6):1295–9.
Kanellos I, Kelpis T, Zaraboukas T, Betsis D. Perineal endometriosis in episiotomy scar with anal sphincter involvement. Tech Coloproctol. 2001;5(2):107–8.
Barisic GI, Krivokapic ZV, Jovanovic DR. Perineal endometriosis in episiotomy scar with anal sphincter involvement: report of two cases and review of the literature. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(6):646–9.
Liang CC, Tsai CC, Chen TC, Soong YK. Management of perineal endometriosis. Int J Gynaecol Obstet. 1996;53(3):261–5.
Kaloo P, Reid G, Wong F. Caesarean section scar endometriosis: two cases of recurrent disease and a literature review. Aust N Z J Obstet Gynaecol. 2002;42(2):218–20.
Scholefield HJ, Sajjad Y, Morgan PR. Cutaneous endometriosis and its association with caesarean section and gynaecological procedures. J Obstet Gynaecol. 2002;22(5):553–4.
Olufowobi O, Sorinola O, Miller SJ, Condie RG. Scar endometrioma: a cause for concern in the light of the rising caesarean section rate. J Obstet Gynaecol. 2003;23(1):86.
Vellido-Cotelo R, Munoz-Gonzalez JL, Oliver-Perez MR, de la Hera-Lazaro C, Almansa-Gonzalez C, Perez-Sagaseta C, et al. Endometriosis node in gynaecologic scars: a study of 17 patients and the diagnostic considerations in clinical experience in tertiary care center. BMC Womens Health. 2015;15:13.
Han L, Zheng A, Wang H. Clear cell carcinoma arising in previous episiotomy scar: a case report and review of the literature. J Ovarian Res. 2016;9:1.
Acknowledgements
The authors thank Dr. Jian Wu for her help with pathological data collection.
Funding
This report was supported by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission: 2019KY496.
Author information
Authors and Affiliations
Contributions
SX and LPS analyzed the data, drafted and wrote the manuscript. SX and WW conducted pathological experiments and analyzed the data. SX and LPS followed the patient and collected the acquired data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Written informed consent was obtained from both of these two patients for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xu, S., Wang, W. & Sun, L.P. Comparison of clear cell carcinoma and benign endometriosis in episiotomy scar - two cases report and literature review. BMC Women's Health 20, 11 (2020). https://doi.org/10.1186/s12905-020-0880-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-020-0880-5






