Abstract
Background
Breathlessness is a prevalent symptom affecting the quality of life (QOL) of Amyotrophic Lateral Sclerosis (ALS) patients. This systematic review explored the interventions for controlling breathlessness in ALS patients, emphasizing palliative care (PALC), non-invasive ventilation (NIV), opioids, and non-pharmacological strategies.
Methods
A comprehensive search of PubMed, Cochrane Library, and Web of Science databases was conducted. Eligibility criteria encompassed adults with ALS or motor neuron disease experiencing breathlessness. Outcomes included QOL and symptom control. Study designs comprised qualitative studies, cohort studies, and randomized controlled trials.
Results
Eight studies were included, most exhibiting low bias risk, comprising one randomized controlled trial, three cohort studies, two comparative retrospective studies, and two qualitative studies (interviews). Most studies originated from Europe, with one from the United States of America. The participants totaled 3423, with ALS patients constituting 95.6%. PALC consultations significantly improved symptom assessment, advance care planning, and discussions about goals of care. NIV demonstrated efficacy in managing breathlessness, with considerations for device limitations. Opioids were effective, though predominantly studied in non-ALS patients. Non-pharmacological strategies varied in efficacy among patients.
Conclusion
The findings underscore the need for individualized approaches in managing breathlessness in ALS. PALC, NIV, opioids, and non-pharmacological strategies each play a role, with unique considerations. Further research, especially ALS-specific self-management studies, is warranted.
Similar content being viewed by others
Introduction
Rationale
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disease, characterized by a combination of both upper and lower motor neuron involvement [1].
Respiratory symptoms, especially breathlessness, are common as the disease evolves, and respiratory failure remains the most frequent cause of death, usually within three to five years from when the first symptoms appear [2, 3]. However, more slowly progressive forms of the illness occur in a small proportion of patients [2, 3]. Currently, there is no cure for ALS and no effective treatment to halt or reverse the progression of the disease [3], so the main purpose is to maximize the quality of life (QOL) of ALS patients [4].
According to the American Thoracic Society, dyspnea is a term used to characterize a subjective experience of breathing discomfort that consists of qualitatively distinct sensations varying in intensity [5]. In ALS patients, symptom assessment is fundamental.
In ALS, breathlessness occurs due to the progressive weakness of the diaphragm and accessory breathing muscles, retained throat secretions, laryngospasm and aspiration while eating or drinking when there is a predominant bulbar involvement [6]. The relationship between pathology and breathlessness perception is inconsistent, explaining why optimizing disease management alone does not guarantee good symptom control [7].
On that account, breathlessness is a complex symptom that can evoke significant distress.
Descriptors related to air hunger are prominent in the language used by patients to describe their dyspnea [8]. Air hunger is considered the most unpleasant quality of dyspnea, eliciting the strongest emotional response [9]. In a prospective, non-randomized study, the anxiety of choking correlated significantly with the intensity of dyspnea in all patients [10].
High-quality evidence is lacking for most topics in ALS management, and many recommendations provided are based on expert consensus among the working group [11].
As remarkable advances in ALS diagnosis and treatment continue to emerge, there will be an increasing need to support patients and families facing complex decisions amidst significant uncertainty and crucial outcomes for both physical and mental well-being [12]. Palliative care (PALC) involvement from the moment of diagnosis is essential to improve symptom control and QOL for patients with ALS and their families [13, 14]. Patients need assurance that despite having incurable conditions, the chronic symptoms they experience during the progression of the disease will be appropriately alleviated [14].
Considering that: (1) ALS is the most common degenerative motor neuron disorder in adult life; and (2) dyspnea is the symptom that most impairs the QOL in these patients; and (3) no robust review on this theme has been done recently; this study aimed to review the literature on breathlessness control in ALS patients.
Objectives
This study aimed to systematically review the literature on non-invasive interventions for controlling breathlessness in ALS patients and their effects on (1) overall symptom control and (2) overall QOL.
Methods
This systematic review followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [15], and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [16].
Eligibility criteria
Participants
Adults with ALS or motor neuron disease experiencing breathlessness. We included all studies for consideration if they included ALS patients as part of the broader sample population, regardless of the proportion of ALS patients in relation to other participants. Participants from any healthcare setting were eligible.
In regard to breathlessness, we accepted the definitions provided by the authors of the included articles without distinguishing whether it was “air hunger” in general or “dyspnea on minor exertion or talking”, tachypnea, orthopnea, among others.
Interventions
PALC, non-invasive ventilation (NIV), pharmacological (opioids) and non-pharmacological treatments specifically targeting breathlessness.
Comparators
Any.
Outcomes
QOL and symptom control (or burden or management). Any definition and any scales of assessment were accepted.
Study design
Any, excluding literature reviews, conference abstracts, book chapters, letters, editorials, commentaries, and academic theses.
We only included articles written in English.
Articles were excluded if they were inaccessible or not subscribed to by our faculty.
Information sources
PubMed, Cochrane Library, and Web of Science databases were systematically searched for relevant articles. No direct contact with authors was made to identify additional sources.
Search strategy
The strategy employed was as follows: (“amyotrophic lateral sclerosis” OR “motor neuron disease” OR “ALS” OR “MND”) AND (dyspnea OR breathlessness) AND (“palliative care” OR ”hospice care” OR “terminal care” OR “end of life”). Filters were set for English language and publication dates from 2011 to 2022. The search concluded on January 3, 2023.
Selection process
The initial screening of articles by title/abstract was performed by the first author. The full text of potentially relevant articles underwent independent eligibility assessments by both authors. Additionally, backward and forward citation searches were conducted, involving the examination of reference lists and the utilization of Scopus and Web of Science to identify articles citing the included studies. Any disagreements related to study selection and data extraction were resolved through discussion and consensus between the authors. To manage references, organize data, and eliminate duplicates, the reference management software EndNote® 20.2.1 for Windows (Clarivate, 2021) was utilized.
Data collection process
A data extraction form was devised within an Excel 16.0® spreadsheet (Microsoft Corporation, 2023). Both authors independently extracted data from the reports, and the data-charting form underwent review until consensus was achieved for all items. No additional processes were employed for obtaining or confirming data from study investigators.
Data items
Data were collected for two primary outcomes: QOL and symptom control. All results aligning with each outcome domain in every study were considered, and we included all measures utilized by the study investigators. Results were compiled if they pertained to the interventions specified in our eligibility criteria.
Additionally, data were gathered for various other variables, including authors, country of origin, year of publication, study design, population, interventions, comparator/control groups, main outcomes, and any noteworthy observations.
Study risk of bias assessment
The appraisal was performed by two independent reviewers. The Joanna Briggs Institute tools were used for qualitative research [17]; and for cohort studies and comparative retrospective chart/registry studies [18]. For randomized controlled trials (RCT), the revised Cochrane RoB2 was employed [19].
Effect measures
In presenting the synthesis of results, we considered all the effect measures utilized by the original authors for each outcome.
Synthesis methods
Given the substantial heterogeneity across studies, a meta-regression analysis was omitted, and results are presented in a narrative format. Synthesis involved grouping studies based on eligible interventions.
Results
Study selection
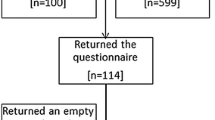
Forty-two articles were initially identified, and after deduplication, 32 references remained. Exclusions included seven articles not meeting the intended study population criteria (although they included ALS patients, dyspnea was not a specific symptom; instead, patients exhibited constitutional symptoms, tiredness, existential problems, etc.); five in non-English languages; and one inaccessible. Additionally, one article was withdrawn, and another had a more recent counterpart. Screening 17 articles for eligibility resulted in nine exclusions due to irrelevant interventions or outcomes from the PALC perspective (e.g., tracheostomy or invasive ventilation). The systematic review ultimately included eight articles, with the selection process depicted in Fig. 1.
Study characteristics
Our review included two qualitative studies with interviews [20, 21], one RCT [22], three cohort studies (two retrospective [23, 24], one prospective [25]), and two comparative retrospective chart/registry studies [26, 27]. The majority of articles originated from Europe, with contributions from the United Kingdom [20, 21], Italy [22], Finland [23], Sweden [24, 26], and France [25]. One article was from the United States of America [27]. The total number of participants was 3423, with 95.6% being ALS patients. Four studies included patients with various comorbidities. Detailed characteristics of the studies are presented in Table 1.
Gysels et al., to understand the lived experience of breathlessness, face-to-face interviewed 48 individuals with various diseases: chronic obstructive pulmonary disease (COPD, n = 18), heart failure (n = 10), ALS (n = 10), and cancer (n = 10) [20].
Simon et al. interviewed 51 people suffering from dyspnea (15 heart failure, 14 COPD, 13 lung cancer, and 9 ALS) [21]. Patients were invited to talk about their experiences with episodic breathlessness, characteristics and triggers for such episodes, impact on daily living and management strategies [21].
Veronese et al., in a non-blinded RCT-parallel arm study with patients affected by neurodegenerative disease (32% with ALS), compared 25 patients immediately referred to PALC to 25 patients with a 16-week wait for referral [22].
Tiirola et al., in a retrospective cohort study, compared symptom prevalence and the prescription rates of opioids, NIV, and oxygen in 32 ALS patients versus 35 individuals with other diseases [23].
Sennfält et al., in a retrospective study, compared 93 ALS patients deceased in 2018–2020 with a cohort of 2224 ALS patients deceased in 2011–2020, focusing on symptom prevalence and NIV use in the last 12 months of life [24].
In a prospective, longitudinal cohort study, Morélot-Panzini et al. examined 41 ALS adult patients eligible for NIV, who received a visit the day before initiating NIV which was prescribed for eight hours during the night [25]. Participants were prompted to choose descriptors for dyspnea across sensory (e.g., ‘I feel air hunger’) and affective dimensions (feeling depressed, anxious, frustrated, or angry) [25].
In a retrospective registry study, Eljas Ahlberg et al. compared 825 deceased ALS patients to 3300 deceased cancer patients, examining topics such as symptom assessment, prescription of as-needed drugs, and communication about the transition to end-of-life care [26].
Mehta et al., in a retrospective chart review, compared nine ALS patients attended by the inpatient PALC team to 15 ALS patients not seen by the team (non-PALC group), exploring topics including goals of care, advance care planning, symptom control, and survival [27].
Risk of bias in studies
Among the seven articles reviewed, all were deemed to be of high quality (refer to Table 2).
The RCT conducted by Veronese et al. [22] exhibited bias in outcome measurement (see Fig. 2). In Veronese’s study, there were systematic errors or inaccuracies in the assessment of study outcomes that could lead to distorted results. The authors mentioned that “some tools were not validated” and “only one evaluation and no crossover could be carried out over time, so we do not know if the improvement in the measured domains is maintained.” These issues compromised the reliability, accuracy, and consistency of outcome assessments in this particular study. Despite the overall compromise in quality, given the low risk in the other four domains and the limited research in this area, we opted to include it in our review.
Results of individual studies
Gysels et al. [20] found that breathlessness varies across conditions, highlighting the need for refined management and tailored interventions for specific patient groups. Nine of 10 ALS patients used positive pressure NIV; however, three reported difficulties [20].
In the study by Simon et al. [21] patients described six main strategies for relieving episodic breathlessness: reducing physical exertion, cognitive and psychological approaches, breathing techniques and positions, air and oxygen, medications and medical devices, and various environmental strategies. The strategies were consistent across disease groups but varied among individuals, often tied to the trigger of the dyspnea episode. These practical strategies, individually applied, can aid in the daily care of patients with episodic breathlessness [21].
In the study by Veronese et al. [22], at the beginning, both groups exhibited similar baseline characteristics; however, after 16 weeks, participants in the fast-track group demonstrated a significant enhancement in both QOL and reduction of breathlessness. Importantly, mortality rates remained comparable between the two arms [22].
Tiirola et al. [23] found that dyspnea, the most common symptom, increased from admission to the last day of life, with no significant differences between ALS patients and those with other non-malignant diseases. Nearly all patients (98%) received as-needed opioids in the last 24 h, and 75% were on regular opioids. Over one-third of ALS patients used NIV in the last 24 h, while patients with other diseases were more frequently given oxygen [23].
Sennfält et al. [24] found that the utilization of regular NIV gradually increased over the last 12 months of life, reaching approximately 50% at the time of death for both ALS patients with spinal and bulbar onset. In the week preceding death, 57 out of 61 ALS patients with anticipated death (a culmination of a slow decline) and 21 out of 29 patients with precipitous death (rapid and unexpected clinical worsening) experienced dyspnea. While dyspnea was effectively managed for most patients, around 29.8% in the “anticipated death” group found only partial or no relief. The lower prevalence of dyspnea in the precipitous death group did not reach statistical significance [24].
Morélot-Panzini et al. [25] found that 36.6% of patients considered ‘lying supine’ as the most severe dyspneic episode within the last 15 days, severely impacting daily life and sleep. However, at the 1-month follow-up, the 27 patients using NIV reported improved episodes, with ‘walking a few steps’ (25.9%) or ‘talking or eating’ (14.8%) as the most unpleasant breathing experiences. NIV initiation significantly reduced sensory dyspnea descriptors but had no significant impact on affective descriptors [25].
Eljas Ahlberg and Axelsson discovered that eight out of ten patients, whether with cancer or ALS, did not undergo symptom assessment (other than pain) within the last week of life [26]. Despite this, 93.3% of ALS patients could communicate less than a week before death, making symptom assessment, including dyspnea, feasible. More ALS patients experienced dyspnea and anxiety compared to cancer patients in the final week. The study found no significant difference in end-of-life communication between the two groups [26].
Mehta et al.‘s study highlights the positive impact of inpatient PALC consultations on ALS patients admitted for non-elective reasons [27]. PALC consultants assessed at least one physical symptom, even when the initial consultation request did not explicitly mention symptom management. The PALC group showed a significant increase in documented Goals of Care compared to the non-PALC group, with 89% and 32% respectively during admission. Moreover, the PALC group had a higher likelihood of having Goals of Care and Advance Care Planning forms documented in their medical records at discharge [27].
Results of synthesis
Most of the articles included were about PALC and NIV in ALS patients.
Palliative care in amyotrophic lateral sclerosis
Patients with ALS were observed to receive less support from specialized PALC teams during the final week of life compared to cancer patients [26]. This discrepancy underscores a potential gap in end-of-life care, as individuals with ALS may experience suboptimal support, characterized by limited validated symptom assessments and prescriptions for as-needed drugs [26]. This situation highlights the necessity for educational interventions aimed at enhancing symptom assessments within PALC, ultimately elevating the quality of care provided to ALS patients.
Notably, PALC consultations played a vital role in improving end-of-life discussions, specifically concerning Goals of Care. Topics such as tracheostomy and the transition to comfort care were significantly enhanced through these consultations [27]. This is particularly crucial considering that, during the end-of-life stage, patients often rely on their families and healthcare providers to communicate their medical wishes [27]. However, a common challenge arises when there is a disparity between patients’ preferences and the medical interventions received, especially in situations where direct communication is not possible. PALC consultations act as a valuable mechanism to ensure alignment with the patient’s preferences, even amid unforeseen acute changes [27].
Despite reservations about the quality of the included RCT, its findings suggest that the involvement of a specialized PALC team positively influences the QOL of patients with neurodegenerative conditions, including ALS [22]. The multifaceted role of the PALC team extends beyond symptom assessment to encompass prescribing medications, providing nursing care, facilitating physical therapies, and offering psychological support [22]. Importantly, it’s noted that the support of a PALC team does not hasten death, emphasizing the beneficial aspects of PALC in improving the overall well-being of individuals facing neurodegenerative conditions like ALS [22].
Non-invasive ventilation in amyotrophic lateral sclerosis
The use of NIV demonstrated effectiveness in managing daily breathing problems when incorporated into the daily routines of ALS patients [20]. As the disease progresses, there is an observed increase in the prevalence of NIV use among ALS patients [24]. In the final stages of the disease, specifically during the last 24 h of life, one-third of ALS patients were reported to have utilized NIV [23].
Research indicates that NIV has positive effects on sensory dyspnea during assisted breathing in ALS patients. However, it is important to note a potential dissociation between the sensory and affective dimensions of dyspnea in this context [25]. This observation emphasizes the nuanced impact of NIV on different aspects of the breathing experience for ALS patients.
Opioid use in amyotrophic lateral sclerosis
Opioids have been employed to manage breathlessness in ALS patients towards the end of life, with notable patterns observed a significant majority of non-cancer ALS patients received as-needed opioids in the last 24 h before death [23]. However, only three out of four ALS patients had regular prescriptions for opioids to address breathlessness [23]. Additionally, less than half of the ALS patient population received opioid treatment specifically for dyspnea [27].
These findings underscore the variability in opioid utilization among ALS patients and highlight potential areas for improvement in the management of breathlessness in this context.
Non-pharmacological treatment in amyotrophic lateral sclerosis
Participants employed various strategies to relieve episodic breathlessness, including reducing activity, changing positions (standing still, sitting, lying down), cognitive techniques (concentration, positive thinking), distraction methods, and the use of breathing techniques (such as, lip breathing, abdominal breathing, leaning forward, or putting the arms up) [21]. Positions for relief varied, with preferences for lying down, standing up, or using pillows. Fresh air, cold temperature, and moistening the mouth were commonly cited as helpful. Some participants found relief in chewing gum or consuming refreshing food [21].
The diversity of effective strategies highlights the importance of individualized support for each patient’s experience.
Discussion
Summary of evidence
In our systematic review (n = 8), we observed that: (1) PALC consultations enhance symptom assessment and control, foster more comprehensive discussions and documentation of goals of care, and advance advanced care planning for ALS patients; (2) NIV effectively manages breathlessness in ALS patients; (3) Opioids prove effective and safe for breathlessness, although most studies are conducted in COPD and cancer patients; (4) The efficacy of non-pharmacological dyspnea control strategies varies among patients with the same disease, emphasizing the need for individualized approaches.
Palliative care in amyotrophic lateral sclerosis
PALC in ALS embraces a comprehensive strategy addressing various dimensions of breathlessness. Physical symptoms necessitate thorough differential diagnosis, along with pharmacological and non-pharmacological management, as well as regular review [28]. The breathing–thinking–functioning model delineates how breathlessness intertwines with psychological, physiological, social, and behavioral factors, perpetuating a cycle that worsens the symptom [7]. This model underscores the significance of Breathlessness Services incorporating tailored support for patients with respiratory diseases, acknowledging the interconnected impact on breathing patterns, mental well-being, and physical functioning [7]. Proactive assessment of physical and psychosocial issues is recommended to reduce the intensity, frequency and need for crisis intervention (unplanned care) [28].
A Consensus Document from the European Association for Palliative Care and the European Academy of Neurology has emphasized the role of PALC for all neurological diseases [28]. Studies on PALC have demonstrated improvements in QOL, symptoms, patient and family satisfaction [28]. PALC therapeutic interventions are hypothesized to indirectly increase survival duration [29].
Conversations about advance care planning [12], and psychosocial and spiritual issues [30] should be initiated early in the disease or whenever the patient inquires. Ongoing discussions about goals of care should be part of routine ALS follow-up [11]. Careful discussion about the wishes of the patient and family – including place of death, funeral arrangements, will, etc. – may be important to ensure that all parties are as prepared as possible [28].
No matter the path, a multidisciplinary PALC approach emphasizing support of patient and caregiver decisions coupled with early open conversations about EOL issues provides patients with dignity in death [1]. The PALC and EOL measure set by the National Quality Forum includes measures within the following domains: comfortable dying, symptom screening, beliefs and documentation of values, care preferences, and treatment preferences [31]. The issues of dying and death should be discussed with patients, particularly as interventions such as NIV and gastrostomy are introduced [32].
Non-invasive ventilation in amyotrophic lateral sclerosis
NIV may improve respiration, QOL [33], and survival duration [29, 33]. Depending on individualized respiratory function, patients must use NIV for prolonged periods of time, ranging from 8 h/day (overnight while sleeping) up to 24 h/day [29]. Patients may initially require slightly longer time to adjust to adaptive ventilator modes, adherence is similar over the longer term [34].
In ALS patients without severe bulbar dysfunction, NIV improved survival (median benefit of 205 days) in a RCT, with maintenance of, and improvement in, QOL [33]. In patients with severe bulbar impairment, NIV improved sleep-related symptoms, but was unlikely to confer a large survival advantage [33]. Moderate-quality evidence from a single RCT also showed that survival and QOL were significantly improved in the subgroup of people with better bulbar function, but not in those with severe bulbar impairment [35].
Recent advancements in NIV technology have led to the development of more sophisticated delivery systems [1]. These devices offer various modes such as bilevel, volume- or pressure-controlled breath delivery, either at specific times or coordinated with spontaneous efforts. Alternatively, they may employ threshold ventilation through adjustable, volume-assured pressure support [34]. Adaptive ventilator settings enable the device to detect and compensate for lapses in baseline ventilation, even in cases of progressive respiratory weakness [34].
Current guidelines primarily focus on NIV initiation and may neglect psychosocial considerations [36]. Optimizing NIV utilization in ALS/MND patients demands a holistic strategy, encompassing specialized multidisciplinary care, patient and family education, caregiver involvement in decision-making, and more. Supportive interventions, consistent monitoring, and continual discussion of patient preferences are equally crucial [36].
The discussion on NIV in ALS requires a nuanced approach based on disease stage [32]. While NIV helps alleviate respiratory symptoms, acknowledging limitations such as potential dependence and challenges like nasal bridge ulceration during continuous use is crucial [37]. Moreover, NIV may reduce overall patient comfort, impede communication, and hinder mobility [29]. Exploring mitigation measures like hydrocolloid dressings and alternate mask systems is crucial. Strategies such as switching between traditional nasal masks and nasal cushion systems, using a mouthpiece during wakefulness (requiring some facial muscle strength), and considering intermittent abdominal pressure ventilation or cuirass ventilators can aid patients [37]. Challenges such as asynchrony and difficulties in mask application, particularly in patients with upper limb involvement, require attention [6, 37]. Acknowledging these aspects ensures informed decision-making regarding the duration and feasibility of NIV use. Recent studies indicate that the benefits of NIV, including increased patient survival and improved mood, outweigh perceived QOL negatives [29].
Considering the close relationship between NIV and oxygen, it is imperative to briefly address the issue of “oxygen need.” Oxygen use should be approached with caution in dyspneic ALS patients, as it may potentially exacerbate hypercapnia [38]. Despite the study by Simon et al. mentioning that ALS patients used oxygen as a strategy to relieve episodic breathlessness [21], and Tiirola et al. finding that oxygen prescription is used in treating dyspnea in ALS patients (albeit less frequently than in individuals with other diseases) [23], oxygen should be regarded as a pharmacological agent in hypoxemic patients and not prescribed based solely on intuitive assumptions of its benefits. Moreover, there is no need to obtain oxygen if this increases the complexity of the endoflife care plan [38].
Healthcare professionals should be prepared for the discontinuation of NIV at the end of life [32], either at the request of a cognitively capable patient or as part of advance care planning, whether through a living will, power of attorney, or advance directive. In such situations, anticipation and effective management of breathlessness and distress symptoms are paramount. The Association for Palliative Medicine of Great Britain and Ireland has issued a comprehensive professional guideline on the withdrawal of long-term ventilation in ALS patients [38]. This guideline elaborates on PALC concepts such as sedation and enhanced symptom control.
Opioid use in amyotrophic lateral sclerosis
In cases where NIV is declined or insufficient, opioids emerge as a safe and effective option for improving dyspnea in ALS patients [13, 39]. Given the overlap in cortical structures activated by pain and air hunger, it is reasonable to consider whether opiates affect central pathways involved in air hunger perception [40] In a blinded RCT with six healthy volunteers, a moderate morphine dose notably relieved laboratory dyspnea, with a minor impact on ventilation. This model established a significant treatment effect, consistent with clinical opioid studies [41]. In managing dyspnea, opioids’ therapeutic effect is enhanced by reducing overall oxygen consumption through alleviating anxiety, fear, and panic [10]. Additionally, decreased respiratory effort reduces oxygen consumption of the respiratory muscles [10].
While existing studies predominantly focus on COPD or cancer patients [13], extrapolating these findings to ALS patients is reasonable.
Advancements in applying PALC to refractory neurological diseases have showcased the efficacy of opioids in ALS [10, 14, 30, 42]. Opioids, as symptomatic treatments for dyspnea, should be readily accessible, even early in the disease progression [43]. Several studies have documented opioid use in ALS patients for controlling breathlessness.
In a prospective study of six bulbar ALS patients in a PALC unit, oral morphine was administered (initial dose: 6.3 ± 7.0 mg), with titration possible in 1 mg increments if needed [10]. Results demonstrated a significant reduction in respiratory rate and dyspnea intensity 120 min post-morphine administration. No significant changes were observed in transcutaneous carbon dioxide partial pressure or oxygen saturation, and oxygen insufflation did not notably decrease dyspnea intensity. Respiratory depression cases were not reported [10].
In a retrospective case-based analysis of 84 ALS patients until death, morphine was administered to all dyspneic patients, primarily orally or via gastrostomy [44]. Most patients (69.9%) did not use mechanical ventilation (MV) until death (no-MV group), while 22.9% utilized only NIV. The final dosage equivalent of morphine in the NIV group was significantly higher (mean 65.7 ± 54.6 mg, range 10–200 mg) than in the no-MV group (mean 31.7 ± 26.9 mg, range 0–120 mg). Additionally, opioid use duration in months was significantly longer in the NIV group compared to the no-MV group (8.37 ± 8.09 vs. 2.70 ± 3.17) [44].
Oral opioid administration is convenient but requires titration due to wide individual variability in enteral drug bioavailability. Studies in ALS patients with breathlessness typically use doses averaging 30 mg or less [10, 45,46,47], with some reaching up to 200 mg [44], or even 520 mg [48]. Most studies demonstrate opioid efficacy in managing breathlessness with manageable adverse effects, primarily obstipation [45]. Titrating dosages against clinical symptoms rarely leads to life-threatening respiratory depression [14, 49]. A systematic review and meta-analysis (n = 67 studies) found no evidence of significant or clinically relevant respiratory adverse effects of opioids for chronic breathlessness [50].
The potential applicability of opioids in ALS merits further investigation.
Non-pharmacological treatment in amyotrophic lateral sclerosis
In our systematic review no studies have explored the effectiveness of self-management strategies in mitigating breathlessness among ALS patients. A recent Cochrane review, encompassing 27 studies and 6008 participants with COPD, revealed that self-management interventions correlate with enhanced QOL, reduced likelihood of respiratory-related hospital admissions, and a low risk of harm [51]. However, the current lack of ALS-specific studies emphasizes the need for dedicated research to explore the efficacy of self-management strategies in enhancing the QOL of individuals with ALS experiencing breathlessness.
In conclusion, the multifaceted nature of breathlessness in ALS necessitates a holistic and personalized approach, integrating pharmacological and non-pharmacological interventions. As Creutzfeldt and Kluger stated, neuropalliative care is an emerging field with a bright future [12], but further research, especially in the context of ALS, is essential to enhance the understanding and implementation of effective strategies to improve the overall well-being of these patients.
Limitations
This study has several limitations. Firstly, it was not registered in a systematic review database. Secondly, despite some studies in our search ostensibly targeting ALS patients, a detailed analysis revealed their exclusion, resulting in the elimination of several articles.
Additionally, certain studies encompassed varied patient groups, making it unclear whether the described interventions were specifically tailored for ALS patients. The majority of the incorporated studies were retrospective, comprising two cohort studies and two chart or registry-based studies. Qualitative studies, though diverse, had limited representation of ALS patients (only 19 participants in two studies). Consequently, the findings may not be readily generalizable.
Regarding NIV interventions, we did not collect data on types of devices, timings of initiation, use duration, modes of ventilation, settings, etc., which would have been useful for comparing results according to our outcomes.
Conclusions
This systematic review offers insights into the multifaceted management of breathlessness in ALS; however, there is still limited available evidence on the optimal management of dyspnea in ALS. PALC consultations emerge as instrumental in enhancing symptom control, advanced care planning, and discussions about goals of care. NIV proves effective but requires a nuanced approach considering device limitations. Opioids, while effective, lack extensive ALS-specific studies. Non-pharmacological strategies exhibit varying efficacy, emphasizing the need for personalized approaches.
Notwithstanding the study’s limitations, notably the lack of ALS-specific self-management studies and the prevalence of retrospective designs, it accentuates the imperative for additional research. Integrating the insights gleaned from this review, in conjunction with sustained research endeavors and the advocacy for educational interventions, will foster a refined and personalized approach, ultimately enhancing the overall well-being of ALS patients grappling with breathlessness.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ALS:
-
amyotrophic lateral sclerosis
- COPD:
-
Chronic Obstructive Pulmonary Disease
- NIV:
-
non-invasive ventilation
- PALC:
-
palliative care
- QOL:
-
quality-of-life
- RCT:
-
randomized controlled trial
References
Niedermeyer S, Murn M, Choi PJ. Respiratory failure in amyotrophic lateral sclerosis. Chest. 2019;155(2):401–8. https://doi.org/10.1016/j.chest.2018.06.035.
Goutman SA, Hardiman O, Al-Chalabi A, Chió A, Savelieff MG, Kiernan MC, et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022;21(5):480–93. https://doi.org/10.1016/S1474-4422(21)00465-8.
National Institute of Neurological Disorders and Stroke. Amyotrophic Lateral Sclerosis (ALS). https://www.ninds.nih.gov/amyotrophic-lateral-sclerosis-als-fact-sheet. Accessed 19 May 2023.
Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, et al. Amyotrophic lateral sclerosis. Lancet. 2022;400(10360):1363–80. https://doi.org/10.1016/S0140-6736(22)01272-7.
Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–52. https://doi.org/10.1164/rccm.201111-2042ST.
Tripodoro VA, De Vito EL. Management of dyspnea in advanced motor neuron diseases. Curr Opin Support Palliat Care. 2008;2(3):173–9. https://doi.org/10.1097/SPC.0b013e32830c9049.
Bausewein C, Schunk M, Schumacher P, Dittmer J, Bolzani A, Booth S. Breathlessness services as a new model of support for patients with respiratory disease. Chron Respir Dis. 2018;15(1):48–59. https://doi.org/10.1177/1479972317721557.
Smith J, Albert P, Bertella E, Lester J, Jack S, Calverley P. Qualitative aspects of breathlessness in health and disease. Thorax. 2009;64(8):713–8. https://doi.org/10.1136/thx.2008.104869.
Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med. 2008;177(12):1384–90. https://doi.org/10.1164/rccm.200711-1675OC.
Clemens KE, Klaschik E. Morphine in the management of dyspnoea in ALS. A pilot study. Eur J Neurol. 2008;15(5):445–50. https://doi.org/10.1111/j.1468-1331.2008.02102.x.
Shoesmith C, Abrahao A, Benstead T, Chum M, Dupre N, Izenberg A, et al. Canadian best practice recommendations for the management of amyotrophic lateral sclerosis. CMAJ. 2020;192(46):E1453–8. https://doi.org/10.1503/cmaj.191721.
Creutzfeldt CJ, Kluger B, Kelly AG, Lemmon M, Hwang DY, Galifianakis NB, et al. Neuropalliative care: priorities to move the field forward. Neurology. 2018;91(5):217–26. https://doi.org/10.1212/WNL.0000000000005916.
Allcroft P. Breathlessness in motor neurone disease: a review of the current strategies and gaps in the evidence. Curr Opin Support Palliat Care. 2014;8(3):213–7. https://doi.org/10.1097/SPC.0000000000000077.
Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS) - revised report of an EFNS task force. Eur J Neurol. 2012;19(3):360–75. https://doi.org/10.1111/j.1468-1331.2011.03501.x.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane; 2023. https://training.cochrane.org/handbook/current. Accessed 19 November 2023).
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–89. https://doi.org/10.1016/j.jclinepi.2021.03.001.
Lockwood C, Munn Z, Porritt K. Qualitative research synthesis: methodological guidance for systematic reviewers utilizing meta-aggregation. Int J Evid Based Healthc. 2015;13(3):179–87. https://doi.org/10.1097/XEB.0000000000000062.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. https://doi.org/10.46658/JBIMES-20-08. Accessed 19 May 2023.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898.
Gysels MH, Higginson IJ. The lived experience of breathlessness and its implications for care: a qualitative comparison in cancer, COPD, heart failure and MND. BMC Palliat Care. 2011;10:15. https://doi.org/10.1186/1472-684X-10-15.
Simon ST, Weingärtner V, Higginson IJ, Benalia H, Gysels M, Murtagh FE, et al. I can breathe again! Patients’ self-management strategies for episodic breathlessness in Advanced Disease, Derived from qualitative interviews. J Pain Symptom Manage. 2016;52(2):228–34. https://doi.org/10.1016/j.jpainsymman.2016.02.016.
Veronese S, Gallo G, Valle A, Cugno C, Chiò A, Calvo A, et al. Specialist palliative care improves the quality of life in advanced neurodegenerative disorders: NE-PAL, a pilot randomised controlled study. BMJ Support Palliat Care. 2017;7(2):164–72. https://doi.org/10.1136/bmjspcare-2014-000788.
Tiirola A, Korhonen T, Surakka T, Lehto JT. End-of-life care of patients with amyotrophic lateral sclerosis and other Nonmalignant diseases. Am J Hosp Palliat Care. 2017;34(2):154–9. https://doi.org/10.1177/1049909115610078.
Sennfält S, Kläppe U, Thams S, Samuelsson K, Press R, Fang F, et al. Dying from ALS in Sweden: clinical status, setting, and symptoms. Amyotroph Lateral Scler Frontotemporal Degener. 2023;24(3–4):237–45. https://doi.org/10.1080/21678421.2022.2096411.
Morélot-Panzini C, Perez T, Sedkaoui K, de Bock E, Aguilaniu B, Devillier P, et al. The multidimensional nature of dyspnoea in amyotrophic lateral sclerosis patients with chronic respiratory failure: air hunger, anxiety and fear. Respir Med. 2018;145:1–7. https://doi.org/10.1016/j.rmed.2018.10.010.
Eljas Ahlberg E, Axelsson B. End-of-life care in amyotrophic lateral sclerosis: a comparative registry study. Acta Neurol Scand. 2021;143(5):481–8. https://doi.org/10.1111/ane.13370.
Mehta AK, Jackson NJ, Wiedau-Pazos M. Palliative Care consults in an inpatient setting for patients with amyotrophic lateral sclerosis. Am J Hosp Palliat Care. 2021;38(9):1091–8. https://doi.org/10.1177/1049909120969959.
Oliver DJ, Borasio GD, Caraceni A, de Visser M, Grisold W, Lorenzl S, et al. A consensus review on the development of palliative care for patients with chronic and progressive neurological disease. Eur J Neurol. 2016;23(1):30–8. https://doi.org/10.1111/ene.12889.
Bond L, Bowen G, Mertens B, Denson K, Jordan K, Vidakovic B, et al. Associations of patient mood, modulators of quality of life, and pharmaceuticals with amyotrophic lateral sclerosis survival duration. Behav Sci (Basel). 2020;10(1):33. https://doi.org/10.3390/bs10010033.
Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1218–26. https://doi.org/10.1212/WNL.0b013e3181bc0141.
National Quality Forum. Palliative and End-of-Life Care 2015–2016. Dec 2016. https://www.qualityforum.org/Palliative_and_End-of-Life_Care_Project_2015-2016.aspx. Accessed 19 May 2023.
National Institute for Health and Clinical Excellence (NICE). Motor neurone disease: assessment and management. NICE guidelines NG 42. London: NICE. 2016. https://www.nice.org.uk/guidance/ng42. Accessed 19 May 2023.
Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5(2):140–7. https://doi.org/10.1016/S1474-4422(05)70326-4.
Sales de Campos P, Olsen WL, Wymer JP, Smith BK. Respiratory therapies for amyotrophic lateral sclerosis: a state of the art review. Chron Respir Dis. 2023;20:14799731231175915. https://doi.org/10.1177/14799731231175915.
Radunovic A, Annane D, Rafiq MK, Brassington R, Mustfa N. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2017;10(10):CD004427. https://doi.org/10.1002/14651858.CD004427.pub4.
Baxter SK, Johnson M, Clowes M, O’Brien D, Norman P, Stavroulakis T, et al. Optimizing the noninvasive ventilation pathway for patients with amyotrophic lateral sclerosis/motor neuron disease: a systematic review. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(7–8):461–72. https://doi.org/10.1080/21678421.2019.1627372.
Polkey MI, Lyall RA, Davidson AC, Leigh PN, Moxham J. Ethical and clinical issues in the use of home non-invasive mechanical ventilation for the palliation of breathlessness in motor neurone disease. Thorax. 1999;54(4):367–71. https://doi.org/10.1136/thx.54.4.367.
Association for Palliative Medicine of Great Britain and Ireland. Withdrawal of assisted ventilation at the request of a patient with motor neurone disease: guidance for professionals. Nov 2015. https://apmonline.org/wp-content/uploads/2016/03/Guidance-with-logos-updated-210316.pdf. Accessed 03 April 2024.
Blackhall LJ. Amyotrophic lateral sclerosis and palliative care: where we are, and the road ahead. Muscle Nerve. 2012;45(3):311–8. https://doi.org/10.1002/mus.22305.
von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, et al. Dyspnea and pain share emotion-related brain network. NeuroImage. 2009;48(1):200–6. https://doi.org/10.1016/j.neuroimage.2009.06.015.
Banzett RB, Adams L, O’Donnell CR, Gilman SA, Lansing RW, Schwartzstein RM. Using laboratory models to test treatment: morphine reduces dyspnea and hypercapnic ventilatory response. Am J Respir Crit Care Med. 2011;184(8):920–7. https://doi.org/10.1164/rccm.201101-0005OC.
Oliver D. Opioid medication in the palliative care of motor neurone disease. Palliat Med. 1998;12(2):113–5. https://doi.org/10.1191/026921698677326556.
Morelot-Panzini C, Bruneteau G, Gonzalez-Bermejo J. NIV in amyotrophic lateral sclerosis: the ‘when’ and ‘how’ of the matter. Respirology. 2019;24(6):521–30. https://doi.org/10.1111/resp.13525.
Morishima R, Shimizu T, Kimura H, Bokuda K, Saotome T, Nakayama Y, et al. High doses of opioids usage for amyotrophic lateral sclerosis patients with non-invasive ventilation. Acta Neurol Belg. 2024;124(1):101–7. https://doi.org/10.1007/s13760-023-02344-5.
Currow DC, McDonald C, Oaten S, Kenny B, Allcroft P, Frith P, Briffa M, Johnson MJ, Abernethy AP. Once-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage. 2011;42(3):388–99. https://doi.org/10.1016/j.jpainsymman.2010.11.021.
Vogt S, Schreiber S, Heinze HJ, Dengler R, Petri S, Vielhaber S. The Dyspnea-ALS-Scale (DALS-15) optimizes individual treatment in patients with amyotrophic lateral sclerosis (ALS) suffering from dyspnea. Health Qual Life Outcomes. 2019;17(1):95. https://doi.org/10.1186/s12955-019-1167-0.
Takahashi K, Murakami F, Komai K, Ishida C, Kato-Motozaki Y. Difference in the care of patients with amyotrophic lateral sclerosis with and without intervention from the palliative care team: observations from a center in Japan. Palliat Med Rep. 2021;2(1):201–6. https://doi.org/10.1089/pmr.2020.0084.
Oliver DJ. Palliative care for patients with motor neurone disease: current challenges. Degener Neurol Neuromuscul Dis. 2016;6:65–72. https://doi.org/10.2147/DNND.S85103.
López-Saca JM, Centeno C. Opioids prescription for symptoms relief and the impact on respiratory function: updated evidence. Curr Opin Support Palliat Care. 2014;8(4):383–90. https://doi.org/10.1097/SPC.0000000000000098.
Verberkt CA, van den Beuken MHJ, Schols JMGA, Datla S, Dirksen CD, Johnson MJ, et al. Respiratory adverse effects of opioids for breathlessness: a systematic review and metaanalysis. Eur Respir J. 2017;50:1701153. https://doi.org/10.1183/13993003.01153-2017.
Schrijver J, Lenferink A, Brusse-Keizer M, Zwerink M, van der Valk PD, van der Palen J, et al. Self-management interventions for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2022;1(1):CD002990. https://doi.org/10.1002/14651858.CD002990.pub4.
Funding
A specific grant was not received for this research from any public, private, or non-profit funding agency.
Author information
Authors and Affiliations
Contributions
This study was conceptualized by CBF and PRP. CBF conducted searches and screening of articles, analyzed data, wrote the manuscript, and approved the final version of the paper. NRC wrote the manuscript, reviewed it, and approved the final paper. PRP designed the review protocol, screened articles, analyzed data, wrote the manuscript, reviewed it, and approved the final paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests in this section.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Filipe, C.B., Carreira, N.R. & Reis-Pina, P. Optimizing breathlessness management in amyotrophic lateral sclerosis: insights from a comprehensive systematic review. BMC Palliat Care 23, 100 (2024). https://doi.org/10.1186/s12904-024-01429-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12904-024-01429-z