Abstract
Background
The presence of a polymicrobial dysbiotic film in direct and constant contact with periodontal tissues initiates the host immune response. Interleukin 18 (IL-18) triggers up-regulates the production of other proinflammatory cytokines (TNF-α, IL-1β, IL-6), creating a vicious cycle that expands the inflammatory and destructive process in the periodontal tissue. A systematic review and meta-analysis was carried out with the main propose to investigate IL-18 expression in different biological samples from subjects with chronic periodontitis.
Methods
The protocol followed PRISMA guidelines and was registered in Open Science Framework (OSF):https://doi.org/10.17605/OSF.IO/BS9GM. A digital search was conducted in the databases PubMed, ScienceDirect, Google Scholar, Web of Science and Dentistry & Oral Sciences Source databases were consulted from March 15th, 2005 to February 10th, 2023. Study quality was assessed using the JBI tool for cross-sectional studies and clinical trials. A meta-analysis was performed using a random/fixed effects model to evaluate the concentration of IL-18 in serum, plasma, saliva, gingival tissue and GCF of exposure group compared to control group.
Results
The search strategy provided a total of 3,156 articles, of which 18 investigations met the inclusion criteria and 15 articles were quantitatively analyzed. The total number of patients studied was 1,275 (682 cases and 593 controls). The meta-analysis revealed significantly elevated IL-18 levels of serum, saliva and GCF of subjects with chronic periodontitis compared to healthy subjects (Serum: SMD = 62.73, 95%CI: 25.43-100.03, Z = 3.29, p = 0.001*; Saliva: SMD = 243.63, 95%CI: 8.68-478.59, Z = 2.03, p = 0.042*; GCF: SMD = 150.26, 95%CI: 56.86-243.66, Z = 3.15, p = 0.02*).
Conclusion
IL-18 levels in serum, saliva and GCF could have the potential to be used as complementary diagnostic tools to the clinical and radiographic parameters in subjects with periodontitis.
Similar content being viewed by others
Introduction
Periodontitis is a chronic immunoinflammatory disease and highly prevalent (62,3%) worldwide [1]. Repeated exposure to a polymicrobial dysbiotic biofilm with predominantly Gram-negative anaerobes such as the periodontopathogenic red complex species; Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola [2], along with other environmental (smoking, nutritional deficiencies) [3, 4] and genetic (single nucleotide polymorphisms/SNPs) [5] risk factors are the main cause of periodontitis development and progression [6]. Clinically it is an asymptomatic condition (which is why patients fail to detect it in its early stages) however, depending on the severity of the disease, there are characteristic signs and symptoms such as gingival erythema and edema, excessive accumulation of dentobacterial plaque, supra and subgingival calculus, gingival recession with root exposure, which increases tooth sensitivity and mobility, presence of bleeding and suppuration spontaneously or with minimal stimulus, which is accompanied by halitosis and metallic taste [7]. The most evident radiographic findings are the widening of the periodontal ligament and the characteristic bone loss in different directions (vertical or horizontal) [8]. In severe cases, this leads to extraction or loss of teeth, which has a significant impact on both the economics and oral health-related quality of life of the individual [9].
The gold standard for the diagnosis of periodontitis continues to be periodontal probing that measures some clinical indicators such as probing depth (PD), clinical attachment level (CAL) and bleeding on probing (BOP), along with radiographic evaluation of the bone surface [10]. However, these tools alone are unable to distinguish the onset and progression of the disease, therefore in recent years, researchers are searching for new molecular biomarkers that contribute to early diagnosis, prognosis and can monitor periodontal diseases [11].
The influence of pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) of red complex species stimulate host cells through Toll-like receptors (TLRs) and consequently activate the nuclear factor kappa B (NF-κB) pathway which, up-regulates the expression of genes encoding a variety of cytokines and chemokines such as tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 23 (IL-23), interleukin 17 (IL-17), as well as interleukin 18 (IL-18) which play an important role in the immunopathogenesis of periodontitis [12, 13].
IL-18, also called interferon gamma-inducible factor or interleukin 1 gamma, is a proinflammatory cytokine, which is encoded by the IL18 gene located at 11q23.1 [14]. Initially, it is synthesized as an inactive precursor (pro-IL-18), which is cleaved by caspase 1 and gives rise to a protein in its mature form, consisting of 193 amino acids and with a molecular weight of 22.36 kDa [15]. IL-18 is produced mainly by keratinocytes, gingival fibroblasts, macrophages and dendritic cells [16]. Once released, IL-18 interacts and binds with its receptor (IL-18R). In this way, IL-18 forms a heterodimer together with the α and β chain of the IL-18 receptor (IL-18Rα and IL-18Rβ) and with the accessory chain of the IL-1R co-receptor (IL-1R3), which allows Toll-IL-1 receptor (TIR) domains to approach and recruit MyD88, resulting in NF-κB activation [17]. Therefore, in addition to triggering the production of interferon gamma (IFN-γ) by T helper type 1 (Th1) cells and IL-17 by Th17 cells contributing to the activation of the IL-23/1L-17 axis, it up-regulates the production of other proinflammatory cytokines, creating a vicious cycle that expands the inflammatory and destructive process in gingival tissue [18, 19].
Since IL-18 expression generally increases in any inflammatory condition, numerous studies have reported differences in IL-18 levels in biological samples such as serum, plasma, saliva, gingival tissue, and gingival crevicular fluid (GCF) of subjects with chronic periodontitis compared to periodontally and systemically healthy subjects [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. However, to date, there is no comprehensive systematic review summarizing the findings of previous studies. Therefore, based on the available scientific evidence, the overall aim of the present study was to investigate IL-18 expression in different biological samples from subjects with chronic periodontitis.
Materials and methods
PICOD and research question
The PICOD algorithm was used:
-
1.
P (Problem): Subjects with chronic periodontitis (Exposure group/EG).
-
2.
I (Intervention): IL-18 levels in serum, plasma, saliva, gingival tissue and GCF.
-
3.
C (Control): Systemically and periodontally healthy subjects (Control group/CG).
-
4.
O (Outcomes): Differences in IL-18 levels in serum, plasma, saliva, gingival tissue and gingival crevicular fluid in EG compared to CG.
-
5.
D (Design): Cross-sectional studies and clinical trials.
Thus, the following research question was posed: Is there an increase in IL-18 levels in serum, plasma, saliva, gingival tissue and GCF in EG compared to CG?
Elegibility criteria
All cross-sectional studies and clinical trials comparing serum, plasma, saliva, saliva, gingival tissue and GCF IL-18 levels in EG compared to CG were accessed. Determination of EG was based on the evaluation of clinical parameters such as PD ≥ 3 mm and CAL ≥ 1 mm, whereas CG was based on the evaluation of systemically healthy subjects, without bone loss and with a PD > 2 mm and CAL < 1 mm. Only studies published in the English language and in peer-reviewed journals were considered. Studies in animal models or cell lines, systematic or narrative reviews, editorials, reviews, and letters to the editor were excluded.
Search strategy and study selection
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [38]. The protocol was registered in Open Science Framework (OSF): https://doi.org/10.17605/OSF.IO/BS9GM.
PubMed, ScienceDirect, Google Scholar, Web of Science and Dentistry & Oral Sciences Source databases were consulted from March 15th, 2005 to February 10th, 2023. The search strategy employed for PubMed was (((((“Interleukin-18“[Mesh]) OR “Serum“[Mesh]) OR “Plasma“[Mesh]) OR “Saliva“[Mesh]) OR “Gingival Crevicular Fluid” [Mesh]) AND “Periodontitis“[Mesh]. For the rest, the keywords used were “IL-18”, “Serum”, “Plasma”, “Saliva”, “Gingival tissue”, “Gingival crevicular fluid” and “Periodontitis”. A secondary hand search was also performed in the following Journals: Periodontology 2000, Journal of Clinical Periodontology, Journal of Periodontology, Journal of Periodontal Research, Journal of Periodontal and Implant Science and International Journal of Periodontics & Restorative Dentistry.
The titles and abstracts of the literature search were then evaluated by two reviewers (M.A.A.S and J. S. B.R) according to previously established eligibility criteria. If the abstract matched the topic, the full text was accessed, while irrelevant articles were excluded. Any disagreement was consulted with a third expert reviewer (A.H) to resolve the debate.
Data items
Initially, data were extracted and tabulated in a Microsoft Office Excel version 16.72 (Microsoft Corporation, Redmond, USA) server sheet by two reviewers independently (M.A.A.S and S.R.S). The data extracted were as follows: First author’s name, year, country, study design, ethics committee approval, journal name, inclusion criteria, exclusion criteria, gender, age, number of EG and CG cases, total study population, periodontal criteria to define the exposure group, clinical parameters evaluated as PS, CAL, plaque index (PI), BOP and radiographic bone loss (RBL), the type of biological sample, type of marker, immunoassay technique used, the mean ± standard deviation (SD), the p value and the main results and conclusions.
Assessment of the risk of bias and quality of the recommendations
To assess the quality and risk of bias of the included cross-sectional studies and clinical trials, the Joanna Brigs Institute (JBI) tool [39] was used. Questions were scored as “Yes”, “No”, “Unclear”, or “Not applicable.” Studies were classified according to their quality, and were placed into three levels; high bias, when the study reached up to 49% of the scores. Moderate bias, when scores were 50–69% and low bias, when scores were > 70%.
Synthesis methods
STATA V.17 (Stata Corp, College Station, TX, USA) was used to perform the meta-analyses. The standardized mean difference (SMD) of IL-18 levels assessed in pg/mL between study groups (exposure vs. control) in different biological samples (serum, plasma, saliva, gingival tissue and GCF) was analyzed using a fixed and/or random effects model (depending on the heterogeneity found). Heterogeneity was estimated using the Chi2 test and quantified with the I2 statistic. Values up to 25% were categorized as low heterogeneity, values between 50% and 75% as medium heterogeneity and values above 75% as high heterogeneity. If moderate-high heterogeneity was detected, a random-effects model was used. A funnel plot together with Egger test was used to evaluate publication bias. A p value ≤ 0.05 was considered statistically significant.
Ethical approval
This study complies with ethical standards.
Results
Study selection
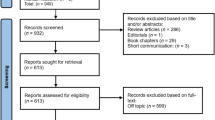
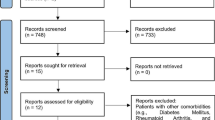
Initially 3,156 articles were found in the five databases, including PubMed (2,080 articles were found), ScienceDirect (178 articles were found), Google Scholar (823 articles were found), Web of Science (3 articles were found), Science and Dentistry & Oral Sciences Source (68 articles were found), and hand searching (4 articles were found). Duplicates were removed and, based on title and abstract, the remaining 2,152 studies were reviewed. After analyzing the full text of the remaining articles, 2,138 records were excluded as irrelevant. A total of 14 articles were assessed for eligibility, plus an additional 4 articles resulting from the hand search. Thus, a total of 18 articles were included for the qualitative analysis and 15 for the quantitative analysis of the present review. Details of the study selection are shown in Fig. 1.
Demographic and clinical features of the studies analyzed
Thirteen articles (72,22%) with a cross-sectional design [20,21,22, 24,25,26, 29, 31,32,33, 35,36,37] and 5 clinical trials (27,77%) [23, 27, 28, 30, 34] were reviewed in this study. The total number of subjects studied in the included investigations was 1,275 of which 682 represented the EG and 593 represented the CG. The ages of the individuals ranged from 25 to 65 years, with a mean age ± SD of 41.52 ± 6.84 years. The 37.33% were male, 47.21% were female and in the remainder (15.45%) sex was not reported [27, 28, 30, 35]. Most of the articles were published after 2009 (15:83.33%) [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. The oldest study was from 2005 [37], and the most recent from 2023 [20]. All studies (100%) were approved by the ethics committee of their respective institutions [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The most frequent exclusion criteria in all the studies analyzed was the presence of systemic diseases (100%) that could be affecting the periodontal condition [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The 18 articles were published in 11 different countries. Five (27.77%) studies were conducted in India [21, 27,28,29, 34], three (16.6%) studies in Brazil [25, 30, 35], two (11.11%) studies in Iran [23, 24] and Turkey [31, 33], and other studies (5.55%) in Bosnia [20], Romania [22], China [26], Mexico [32], Australia [36] and USA [37]. In addition, the journals of publication are shown (Table 1).
The most frequently evaluated clinical parameters were PD (88.88%) [20, 24, 26,27,28,29,30,31,32,33,34,35, 37] and CAL (77.77%) [20,21,22,23,24, 26,27,28, 30,31,32,33,34,35]. Regarding the analysis of the different biological samples for the determination of IL-18 levels, ten (55.55%) studies evaluated GCF samples [21,22,23, 27, 28, 30, 33,34,35,36], six (33.33%) studies serum samples [20, 25, 26, 28, 32, 36], four (22.22%) studies saliva samples [24, 26, 29, 31], two (11.11%) studies plasma samples [29, 31] and gingival tissue [32, 37]. In addition, only one (5.55%) study used Bead-based Flow Cytometric Assay [20], whereas, the rest (94.44%) used ELISA technique [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] (Table 2).
Meta-analysis of IL-18 in serum samples
Six studies [20, 25, 26, 28, 32, 36] compared serum IL-18 levels between EG (n = 309) and CG (n = 217). The results of the meta-analysis indicated a SMD = 62.73(25.43-100.03, p = 0.001*) demonstrating a significant increase of this cytokine in serum samples from EG compared to CG. Based on the chi-square test, there was no evidence of heterogeneity between studies (I2 = 41.3%, p = 0.182), noting that, study heterogeneity was low (Fig. 2, panel A).
Meta-analysis of IL-18 in plasma samples
Two studies [29, 31] compared plasma IL-18 levels between EG (n = 62) and CG (n = 41). The meta-analysis results indicated a SMD = 236.67(-36.69-510.02), p = 0.090) demonstrating a non-significant increase of this cytokine in plasma samples from EG compared to CG. Based on the chi-square test, there was no evidence of heterogeneity between studies (I2 = 0.0%, p = 0.994), noting that, study heterogeneity was low (Fig. 2, panel B).
Meta-analysis of IL-18 in saliva samples
Four studies [24, 26, 29, 31] compared saliva IL-18 levels between EG (n = 112) and CG (n = 91). The meta-analysis results indicated a SMD = 243.63(8.68-478.59), p = 0.042) demonstrating a significant increase of this cytokine in saliva samples from EG compared to CG. Based on the chi-square test, there was evidence of heterogeneity among the studies (I2 = 89.6%, p = 0.000*), noting that, the heterogeneity of the studies was high, therefore a random effects model was used to pool the primary results (Table 3). The funnel plot shown the asymmetry and possibility of publication bias. However, Egger’s test (t = 2.50, p = 0.129) showed no evidence of bias (Fig. 2, panel C and Fig. 3).
Meta-analysis of IL-18 in gingival tissue samples
Two studies [32, 37] compared IL-18 levels in gingival tissue between EG (n = 108) and CG (n = 51). The results of the meta-analysis indicated a SMD = 206.82(-172.37-586.00), p = 0.285) demonstrating a non-significant increase of this cytokine in gingival tissue samples from EG compared to CG. Based on the chi-square test, there was evidence of heterogeneity among the studies (I2 = 99.1%, p = 0.000), noting that, study heterogeneity was high, therefore a random effects model was used to pool the primary results (Fig. 2, panel D).
Meta-analysis of IL-18 in GCF samples
Eight studies [21,22,23, 27, 28, 30, 33,34,35,36] compared IL-18 levels in GCF between EG (n = 173) and CG (n = 184). The results of the meta-analysis indicated a SMD = 150.26(56.86-243.66), p = 0.02*) demonstrating a significant increase of this cytokine in GCF samples from the EG compared to the CG. Based on the chi-square test, there was evidence of heterogeneity among the studies (I2 = 98.1%, p = 0.000), noting that, the heterogeneity of the studies was high, therefore a random effects model was used to pool the primary results (Table 3). The funnel plot shown the asymmetry and possibility of publication bias. However, Egger’s test (t = 2.30, p = 0.06) showed no evidence of bias (Fig. 2, panel E and Fig. 3).
Quality assessment and risk of bias
The JBI checklist was used to assess the quality of cross-sectional studies and clinical trials. According to the established criteria, 11 (61.11%) studies had a low risk of bias [20, 21, 24, 26, 29, 31,32,33, 35,36,37], 6 (33.33%) studies a moderate risk of bias [23, 25, 27, 28, 30, 34] and only 1 (5.55%) a high risk of bias [22] (Figs. 4 and 5).
Discussion
This study investigated IL-18 levels in serum, plasma, saliva, gingival tissue and GCF samples of subjects with chronic periodontitis compared to periodontally healthy subjects. A total of 18 articles published in 11 different countries were analyzed. Overall, the most important findings of the present meta-analysis showed a significant increase in IL-18 levels in serum, saliva and GCF of EG compared to CG. On the other hand, of the two studies that evaluated IL-18 levels in plasma, one of them showed inverse results (> 0.05) [31], while, in gingival tissue, another study showed no significant differences (> 0.05) [32], therefore, for these biological samples the results were inconclusive.
The presence of a polymicrobial dysbiotic film in direct and constant contact with periodontal tissues initiates the host immune response, which is constituted by an innate immune response, which provides the initial defense against periodontopathogens and its different components are; the gingival barrier, antimicrobial peptides produced by oral keratinocytes and other cells of the gingival sulcus, phagocytic cells such as macrophages (MΦ) and neutrophils, dendritic cells (DC), natural killer (NK) cells and other innate lymphoid cells [40]. B and T cells, on the other hand, regulate the adaptive immune response [41]. Both responses are closely related to each other, and among other mechanisms, lead to the production and release of inflammatory molecules such as TNF-α IL-1β, IL-6 and IL-18 which up-regulate the expression of matrix metalloproteases (MMPs) such as aMMP-8, MMP-9, MMP-13 which contribute in the degradation of the extracellular matrix of the gingival tissue and on the other hand, these proinflammatory cytokines increase the expression of RANKL, which binds with its receptor (RANK) and activates intracellular signaling pathways that initiate the process of osteoclastogenesis, leading to alveolar bone loss [42].
Proinflammatory cytokines reach oral biofluids (GCF and saliva) and can be detected through different techniques such as enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, in situ hybridization, polymerase chain reaction (PCR), among others [43]. Therefore, there is increasing evidence in the literature indicating associations between these inflammatory mediators (TNF-α IL-1β, IL-6, IL-8, IL-23/IL-17, IL-18) and periodontitis. In this sense, most authors concluded that these molecules could be considered as potential biomarkers to diagnose, prognosticate and monitor periodontal diseases [19, 44,45,46].
In relation to IL-18, its role has been demonstrated in some inflammatory diseases such as Crohn’s disease, celiac disease, type 2 diabetes mellitus, obesity, rheumatoid arthritis, systemic lupus erythematosus, and graft-versus-host disease [13]. For example, patients with inflammatory bowel disease and periodontitis have been associated with higher serum levels of IL-8 and IL-18 compared to subjects without coexistence of both diseases [47]. Also, in patients with celiac disease, whose main complication is xerostomia, leading to dental caries and periodontal disease, the mean levels of IL-6, IL-18 and IL-21 in serum and saliva were higher compared to that of their control group [48]. On the other hand, it has been shown that in subjects with type 2 diabetes mellitus and periodontitis the serum and salivary levels of IL-17 and IL-18 were not different compared to systemically and periodontally healthy subjects. However, a statistically significant association was found between serum IL-18 levels and glycosylated hemoglobin (HbA1c), therefore, IL-18 levels reflected the patient’s glycemic status and not the periodontal status [49]. Likewise, it has been shown that obesity could hinder the improvement of periodontal clinical and immunological parameters after non-surgical therapy in patients with diabetes mellitus and periodontitis [50].
On the other hand, several studies have explored the role of IL-18 in periodontal diseases. In summary, it has been shown that IL-18 is mainly released from DC and acts synergistically with IL-12 on NK cells to trigger the production of IFN-γ from Th1 cells and IL-17 from Th17 cells, as well as exacerbating the inflammatory process by up-regulating other cytokines such as TNF-α, IL-1β and CX3CL1 that induce MMP-9 production and recruitment of osteoclast precursor cells, leading to further inflammation and destruction of periodontal tissues [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37].
The activity and function of cytokines at the systemic level is evaluated by measuring their concentrations in serum and plasma, because the components of these biofluids reflect the inflammatory condition of the host in periodontal disease, therefore, it is plausible to evaluate IL-18 levels in these biological samples to investigate the pathogenesis of periodontitis [20, 25, 26, 28, 32, 36]. In this meta-analysis, it was shown that the serum IL-18 level in subjects with chronic periodontitis was significantly higher than that in healthy subjects, this finding is in agreement with the results of previous studies.
Cicmil et al., 2023 demonstrated an increase in biochemical (C-reactive protein, fibrinogen, triglycerides, total cholesterol and LDL-cholesterol), hematological (fibrinogen, systolic, diastolic blood pressure, right and left intima media thickness), and IL-8 and IL-18 levels in subjects with periodontitis compared to healthy subjects. In addition, the authors found a positive correlation between the levels of these cytokines with gingival index (GI), BOP, PD and CAL. Thus, these results suggest that dyslipidemia and an altered periodontal condition could be risk factors for the development of other serious systemic disease such as subclinical atherosclerosis [20]. Likewise, studies by Zhang et al., 2021, Tsuneto et al., 2019, Wang et al., 2019 and Sánchez-Hernández et al., 2012 showed a significant increase in IL-18 levels in subjects with periodontitis compared to periodontally healthy subjects. These findings suggest that this cytokine is involved in chronic inflammation and tissue destructive process, in subjects with periodontitis. Therefore, the authors conclude that serum IL-18 concentrations could be used as biomarkers to predict disease development [25, 26, 32, 50]. On the other hand, Nair et al., 2016 demonstrated in their study that serum IL-18 levels are directly proportional to the increase in the level of inflammation in periodontal tissues, while a decrease in the level of inflammation after non-surgical periodontal therapy leads to a reduction in the levels of this cytokine [28]. Only one study [36] reported very low levels of IL-18 in serum samples.
On the other hand, this meta-analysis showed that there were no significant differences in IL-18 levels between subjects with chronic periodontitis and healthy controls, as analyzed in plasma and gingival tissue. Banu et al., 2014 demonstrated increased levels of TLR-4 and IL-18 in plasma samples from subjects with periodontitis compared to healthy subjects. These results inform on the systemic immune response against periodontopathogenic bacteria. In addition, high levels of TLR-4 could play an important role in the production of other proinflammatory cytokines, wich would produce greater tissue damage [29]. However, Ozçaka et al., 2011 found no statistically significant difference between plasma levels of this cytokine in both EG compared to CG [31]. Sánchez-Hernández et al., 2011 [32] found no difference between the levels of this cytokine in gingival tissue samples, while, Johnson et al., 2005 [37] found increased levels of IL-18 in gingival tissue samples from the EG compared to the CG.
Saliva can be collected easily, noninvasively and painlessly. It consists of a wide variety of biological components such as proteins, carbohydrates, lipids, DNA, RNA, microorganisms and metabolites that could be considered as potential markers for the diagnosis of different oral and systemic diseases [51]. In this meta-analysis, it was shown that the salivary IL-18 level in subjects with chronic periodontitis was significantly higher than that in healthy subjects, this finding is in agreement with the results of previous studies. Vahabi et al., 2019, Wang et al., 2019, Banu et al., 2014 and Ozçaka et al., 2011 found increased levels of IL-18 in saliva of subjects with chronic periodontitis compared to healthy individuals. In addition, Wang et al., 2019 demonstrated a positive correlation between the levels of this cytokine with PI, GI, PD and CAL [26].
Finally, the qualitative analysis revealed that, of the five biological samples previously analyzed, GCF was the most reported [21,22,23, 27, 28, 30, 33,34,35,36]. In pathological conditions, GCF corresponds to an inflammatory exudate made up of a complex mixture of substances derived from serum, leukocytes, proteins, structural cells of the periodontium and oral bacteria [52]. The results of the meta-analysis demonstrated that IL-18 levels in GCF of subjects with chronic periodontitis were significantly higher than that of healthy subjects, this finding is in agreement with the results of previous studies. Nair et al., 2022, Surlin et al., 2021, Shahbeik et al., 2021, Mahajani et al., 2017, Nair et al., 2016, Campos et al., 2012, Türkoğlu et al., 2009, Pradeep et al., 2009, Figueredo et al., 2008 and Orozco et al., 2006 [21,22,23, 27, 28, 30, 33,34,35,36] demonstrated that IL-18 levels in GCF were increased in EG compared to CG. A positive correlation between IL-18 levels with periodontal clinical parameters (PI, GI, PD, CAL) was also demonstrated. The increase of IL-18 in GCF could be due to the damage produced by P. gingivalis and other periodontopathogenic species in the gingival sulcus cells. In addition, it is important to mention that high levels of locally secreted IL-18 (saliva and GCF) could increase circulating IL-18, indicating that periodontal inflammation could up-regulate serum levels of this cytokine in subjects with chronic periodontitis [20, 25, 26, 28, 32, 36]. The authors concluded that IL-18 levels present in GCF have the potential to be considered as biomarkers of periodontal inflammation and destruction. Importantly, variations in GCF flow and different collection methods during sampling may contribute to data heterogeneity, with absorbent paper strips being the most common method [19].
Limitations
The present systematic review and meta-analysis has provided valuable information on the association between IL-18 levels and periodontitis, however, it had some limitations:
-
Lack of sufficient included studies. Mostly studies with a cross-sectional design were analyzed. Only one clinical trial was not added to the meta-analysis due to lack of data. Mostly studies with a cross-sectional design were analyzed.
-
The sample size could be larger, which could increase statistical power and make inferences in the general population.
-
The heterogeneity of the data based on age, gender, inflammatory body conditions, presence of prosthetic restorations, polymicrobial dysbiosis, lipid, hormonal and hematological profile, sampling method and type of technique used for subsequent analysis, could affect this heterogeneity, so the results should be interpreted with caution.
-
Follow-up studies with a larger sample size are suggested, which would allow stratification of the study population based on the new classification of periodontal and peri-implant diseases with more precise criteria and a complete consideration of the influential variables for statistical analysis.
Conclusions
IL-18 plays an important role in the immunopathogenesis of periodontitis. IL-18 levels in serum, saliva and GCF could have the potential to be used as complementary diagnostic tools to the clinical and radiographic parameters considered today as the gold standard in “periodontal diagnosis”. Therefore, future studies in this regard are recommended to ensure their specificity and sensitivity as biomarkers.
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Abbreviations
- IFN-γ:
-
Interferon gamma
- IL-1β:
-
Interleukin- 1 beta
- TNF-α:
-
Tumor necrosis factor- alpha
- IL-6:
-
Interleukin- 6
- IL-8:
-
Interleukin- 8
- IL-17:
-
Interleukin- 17
- IL-18:
-
Interleukin- 18
- IL-23:
-
Interleukin- 23
- CX3CL1:
-
C-X3-C motif ligand 1
- TLR-4:
-
Toll like receptor 4
- aMMP-8:
-
Active matrix metalloproteinase 8
- MMP-9:
-
Matrix metalloproteinase 9
- MMP-13:
-
Matrix metalloproteinase 13
References
Trindade D, Carvalho R, Machado V, Chambrone L, Mendes JJ, Botelho J. Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta-analysis of epidemiological studies. J Clin Periodontol. 2023;50(5):604–26. https://doi.org/10.1111/jcpe.13769.
Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. https://doi.org/10.1034/j.1600-0757.2002.280102.x.
Apatzidou DA. The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Periodontol 2000. 2022;90(1):45–61. https://doi.org/10.1111/prd.12449.
Ustianowski Ł, Ustianowska K, Gurazda K, Rusiński M, Ostrowski P, Pawlik A. The role of Vitamin C and Vitamin D in the Pathogenesis and therapy of Periodontitis-Narrative Review. Int J Mol Sci. 2023;24(7):6774. https://doi.org/10.3390/ijms24076774.
Ahmad P, Siqueira WL. Polymorphism of salivary proteins and risk of periodontal diseases: a systematic review and meta-analysis of clinical studies. J Dent. 2024;141:104804. https://doi.org/10.1016/j.jdent.2023.104804.
Darby I. Risk factors for periodontitis & peri-implantitis. Periodontol 2000. 2022;90(1):9–12. https://doi.org/10.1111/prd.12447.
Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World workshop on the classification of Periodontal and Peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):S237–48. https://doi.org/10.1002/JPER.17-0733.
Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159–72. https://doi.org/10.1002/JPER.18-0006. Erratum in: J Periodontol. 2018;89(12):1475.
Booth J, Erwin J, Burns L, Axford N, Horrell J, Wheat H, et al. A scoping review of the oral health status, oral Health behaviours and interventions to improve the Oral Health of Children and Young People in Care and Care leavers. Dent J (Basel). 2024;12(2):38. https://doi.org/10.3390/dj12020038.
Kwon T, Lamster IB, Levin L. Current concepts in the management of Periodontitis. Int Dent J. 2021;71(6):462–76. https://doi.org/10.1111/idj.12630. Epub 2021 Feb 19.
Kalsi AS, Moreno F, Petridis H. Biomarkers associated with periodontitis and peri-implantitis: a systematic review. J Periodontal Implant Sci. 2021;51(1):3–17. https://doi.org/10.5051/jpis.1902840142.
Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019;11(3):30. https://doi.org/10.1038/s41368-019-0064-z.
Li Y, Chen Y, Cai G, Ni Q, Geng Y, Wang T, Bao C, Ruan X, Wang H, Sun W. Roles of trained immunity in the pathogenesis of periodontitis. J Periodontal Res. 2023;58(5):864–73. https://doi.org/10.1111/jre.13158.
Landy E, Carol H, Ring A, Canna S. Biological and clinical roles of IL-18 in inflammatory diseases. Nat Rev Rheumatol. 2024;20(1):33–47. https://doi.org/10.1038/s41584-023-01053-w.
Kim S, Yu H, Azam T, Dinarello CA. Interleukin-18 binding protein (IL-18BP): a long Journey from Discovery to Clinical Application. Immune Netw. 2024;24(1):e1. https://doi.org/10.4110/in.2024.24.e1.
Yasuda K, Nakanishi K, Tsutsui H. Interleukin-18 in Health and Disease. Int J Mol Sci. 2019;20(3):649. https://doi.org/10.3390/ijms20030649.
Ihim SA, Abubakar SD, Zian Z, Sasaki T, Saffarioun M, Maleknia S, Azizi G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: biological role in induction, regulation, and treatment. Front Immunol. 2022;13:919973. https://doi.org/10.3389/fimmu.2022.919973.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. https://doi.org/10.1038/s41577-019-0165-0.
Alarcón-Sánchez MA, Guerrero-Velázquez C, Becerra-Ruiz JS, Rodríguez-Montaño R, Avetisyan A, Heboyan A. IL-23/IL-17 axis levels in gingival crevicular fluid of subjects with periodontal disease: a systematic review. BMC Oral Health. 2024;24(1):302. https://doi.org/10.1186/s12903-024-04077-0.
Cicmil S, Cicmil A, Pavlic V, Krunić J, Sladoje Puhalo D, Bokonjić D, Čolić M. Periodontal Disease in Young adults as a risk factor for subclinical atherosclerosis: a clinical, biochemical and immunological study. J Clin Med. 2023;12(6):2197. https://doi.org/10.3390/jcm12062197.
Nair V, Grover V, Arora S, Das G, Ahmad I, Ohri A, Sainudeen S, Saluja P, Saha A. Comparative Evaluation of Gingival Crevicular Fluid Interleukin-17, 18 and 21 in Different Stages of Periodontal Health and Disease. Med (Kaunas). 2022;58(8):1042. https://doi.org/10.3390/medicina58081042.
Surlin P, Lazar L, Sincar C, Gheorghe DN, Popescu DM, Boldeanu VM, et al. NLRP3 inflammasome expression in Gingival Crevicular Fluid of patients with Periodontitis and Chronic Hepatitis C. Mediators Inflamm. 2021;2021:6917919. https://doi.org/10.1155/2021/6917919.
Shahbeik S, Taleghani F, Sattari M, Mahvash Mohammadi M, Moravej M. Evaluation of NLRP3 and IL-18 levels after Periodontal Therapy. Iran J Allergy Asthma Immunol. 2021;20(6):764–70. https://doi.org/10.18502/ijaai.v20i6.8028.
Vahabi S, Yadegari Z, Pournaghi S. The comparison of the salivary concentration of interleukin-17 and interleukin-18 in patients with chronic periodontitis and healthy individuals. Dent Res J (Isfahan). 2020;17(4):280–6.
Tsuneto PY, de Souza VH, de Alencar JB, Zacarias JMV, Silva CO, Visentainer JEL, Sell AM. IL18 Polymorphism and Periodontitis Susceptibility, Regardless of IL12B, MMP9, and Smoking Habits. Mediators Inflamm. 2019;2019:9585964. https://doi.org/10.1155/2019/9585964
Wang F, Guan M, Wei L, Yan H. IL-18 promotes the secretion of matrix metalloproteinases in human periodontal ligament fibroblasts by activating NFκB signaling. Mol Med Rep. 2019;19(1):703–10. https://doi.org/10.3892/mmr.2018.9697. Epub 2018 Nov 26. PMID: 30483730.
Mahajani MJ, Jadhao VA, Wankhade PS, Samson E, Acharya VD, Tekale PD. Effect of Periodontal Therapy on Crevicular Fluid Interleukin-18 level in Periodontal Health and Disease in Central Maharashtra (India) Population. J Contemp Dent Pract. 2017;18(11):1085–9. https://doi.org/10.5005/jp-journals-10024-2180.
Nair V, Bandyopadhyay P, Kundu D, Das S. Estimation of interleukin-18 in the gingival crevicular fluid and serum of Bengali population with periodontal health and disease. J Indian Soc Periodontol. 2016;20(3):260–4. https://doi.org/10.4103/0972-124X.183098.
Banu S, Jabir NR, Mohan R, Manjunath NC, Kamal MA, Kumar KR, Zaidi SK, Khan MS, Tabrez S. Correlation of toll-like receptor 4, interleukin-18, transaminases, and uric acid in patients with chronic periodontitis and healthy adults. J Periodontol. 2015;86(3):431–9. https://doi.org/10.1902/jop.2014.140414.
de Campos BO, Fischer RG, Gustafsson A, Figueredo CM. Effectiveness of non-surgical treatment to reduce il-18 levels in the gingival crevicular fluid of patients with periodontal disease. Braz Dent J. 2012;23(4):428–32. https://doi.org/10.1590/s0103-64402012000400020.
Ozçaka O, Nalbantsoy A, Buduneli N. Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. J Periodontal Res. 2011;46(5):592–8. https://doi.org/10.1111/j.1600-0765.2011.01377.x.
Sánchez-Hernández PE, Zamora-Perez AL, Fuentes-Lerma M, Robles-Gómez C, Mariaud-Schmidt RP, Guerrero-Velázquez C. IL-12 and IL-18 levels in serum and gingival tissue in aggressive and chronic periodontitis. Oral Dis. 2011;17(5):522–9. https://doi.org/10.1111/j.1601-0825.2011.01798.x.
Türkoğlu O, Emingil G, Kütükçüler N, Atilla G. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J Periodontol. 2009;80(6):969–76. https://doi.org/10.1902/jop.2009.080532.
Pradeep AR, Hadge P, Chowdhry S, Patel S, Happy D. Exploring the role of Th1 cytokines: interleukin-17 and interleukin-18 in periodontal health and disease. J Oral Sci. 2009;51(2):261–6. https://doi.org/10.2334/josnusd.51.261.
Figueredo CM, Rescala B, Teles RP, Teles FP, Fischer RG, Haffajee AD, Socransky SS, Gustafsson A. Increased interleukin-18 in gingival crevicular fluid from periodontitis patients. Oral Microbiol Immunol. 2008;23(2):173–6. https://doi.org/10.1111/j.1399-302X.2007.00408.x.
Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral Microbiol Immunol. 2006;21(4):256 – 60. https://doi.org/10.1111/j.1399-302X.2006.00292.x. PMID: 16842511.
Johnson RB, Serio FG. Interleukin-18 concentrations and the pathogenesis of periodontal disease. J Periodontol. 2005;76(5):785 – 90. https://doi.org/10.1902/jop.2005.76.5.785. PMID: 15898940.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29:372n71. https://doi.org/10.1136/bmj.n71.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI, 2020. https://synthesismanual.jbi.global
Wu Q, Zhang W, Lu Y, Li H, Yang Y, Geng F, Liu J, Lin L, Pan Y, Li C. Association between periodontitis and inflammatory comorbidities: the common role of innate immune cells, underlying mechanisms and therapeutic targets. Int Immunopharmacol. 2024;128:111558. https://doi.org/10.1016/j.intimp.2024.111558. Epub 2024 Jan 23.
Li Y, Li X, Guo D, Meng L, Feng X, Zhang Y, Pan S. Immune dysregulation and macrophage polarization in peri-implantitis. Front Bioeng Biotechnol. 2024;12:1291880. https://doi.org/10.3389/fbioe.2024.1291880.
Tsukasaki M. RANKL and osteoimmunology in periodontitis. J Bone Min Metab. 2021;39(1):82–90. https://doi.org/10.1007/s00774-020-01165-3. Epub 2020 Oct 17.
Rathnayake N, Gieselmann DR, Heikkinen AM, Tervahartiala T, Sorsa T. Salivary Diagnostics-Point-of-care diagnostics of MMP-8 in dentistry and medicine. Diagnostics (Basel). 2017;7(1):7. https://doi.org/10.3390/diagnostics7010007.
Boronat-Catalá M, Catalá-Pizarro M, Bagán Sebastián JV, Almehmadi AH, Alghamdi F. Salivary and crevicular fluid interleukins in gingivitis. J Clin Exp DentArch Oral Biol. 20142018;6(2):e175–9. https://doi.org/10.4317/jced.51403.
Madureira DF, De Abreu Lima L, Costa I, Lages GC, Martins EMB, Aparecida Da Silva CC. Tumor necrosis factor-alpha in Gingival Crevicular Fluid as a diagnostic marker for Periodontal diseases: a systematic review. J Evid Based Dent Pract. 2018;18(4):315–31. https://doi.org/10.1016/j.jebdp.2018.04.001.
de Mello-Neto JM, Nunes JGR, Tadakamadla SK, da Silva Figueredo CM. Immunological traits of patients with Coexistent Inflammatory Bowel Disease and Periodontal Disease: a systematic review. Int J Environ Res Public Health. 2021;18(17):8958. https://doi.org/10.3390/ijerph18178958.
Ajdani M, Mortazavi N, Besharat S, Mohammadi S, Amiriani T, Sohrabi A, Norouzi A, Edris G. Serum and salivary tissue transglutaminase IGA (tTG-IGA) level in celiac patients. BMC Gastroenterol. 2022;22(1):375. https://doi.org/10.1186/s12876-022-02456-x.
Techatanawat S, Surarit R, Chairatvit K, Khovidhunkit W, Roytrakul S, Thanakun S, Kobayashi H, Khovidhunkit SP, Izumi Y. Salivary and serum interleukin-17A and interleukin-18 levels in patients with type 2 diabetes mellitus with and without periodontitis. PLoS ONE. 2020;15(2):e0228921. https://doi.org/10.1371/journal.pone.0228921.
Pepelassi E, Xynogala I, Perrea D, Pantopoulou A, Agrogiannis G, Vrotsos I. The effect of experimental periodontitis, experimental diabetes and their combination on the serum levels of adiponectin, leptin, IL-6, IL-18, MCP-1, RANTES and sICAM-1 in rats. J Int Acad Periodontol. 2020;22(1):1–10.
Zhang X, He S, Lu W, Lin L, Xiao H. Glycogen synthase kinase-3β (GSK-3β) deficiency inactivates the NLRP3 inflammasome-mediated cell pyroptosis in LPS-treated periodontal ligament cells (PDLCs). Vitro Cell Dev Biol Anim. 2021;57(4):404–14. https://doi.org/10.1007/s11626-021-00583-5.
Melguizo-Rodríguez L, Costela-Ruiz VJ, Manzano-Moreno FJ, Ruiz C, Illescas-Montes R. Salivary biomarkers and their application in the diagnosis and monitoring of the most common oral pathologies. Int J Mol Sci. 2020;21(14):5173. https://doi.org/10.3390/ijms21145173.
Alarcón-Sánchez MA, Heboyan A, Fernandes GVO, Castro-Alarcón N, Romero-Castro NS. Potential impact of prosthetic biomaterials on the Periodontium: a Comprehensive Review. Molecules. 2023;28(3):1075. https://doi.org/10.3390/molecules28031075.
Acknowledgements
None.
Funding
No external funding was received.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.A.A.-S.; methodology, M.A.A.-S-.; software, M.A.A.-S.; validation, M.A.A.-S, A.H. and J.S.B.-R.; formal analysis, M.A.A.-S, N.S.R.-C, J.S.B.-R, and S.R.S.; investigation, M.A.A.-S.; resources, M.A.A.-S.; data curation, M.A.A.-S.; writing—original draft preparation, M.A.A.-S, N.S.R.-C, J.S.B.-R, and S.R.S.; writing—review and editing, M.A.A.-S, J.S.B.-R.; and A.H; visualization M.A.A.-S, N.S.R.-C, J.S.B.-R, A.H. and S.R.S.; supervision, M.A.A.-S, N.S.R.-C, J.S.B.-R, and S.R.S.; project administration, M.A.A.-S. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alarcón-Sánchez, M.A., Romero-Castro, N.S., Becerra-Ruiz, J.S. et al. Increased of IL-18 levels are associated with periodontitis: a systematic review and meta-analysis. BMC Oral Health 24, 981 (2024). https://doi.org/10.1186/s12903-024-04747-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04747-z