Abstract
Background
The present study aimed to assess how a concentrated growth factor (CGF) injection affects the rate of orthodontic tooth movement in rabbits.
Methods
This experimental investigation employed a split-mouth configuration. Before orthodontic mesialization of the maxillary first molars, CGF was prepared and administered using submucosal injections on the buccal and palatal sides of the maxillary first molars in one randomly assigned quadrant. The opposite quadrant was used as a control. The study examined four time points:1, 2, 3, and 4 weeks. The measurement of tooth movement was conducted at each follow-up point using a digital caliper. The rabbits were euthanized, and their maxillary segments, specifically the maxillary first molars, were studied histologically to identify any alterations occurring on both the tension and compression sides.
Results
Significant tooth movement was observed in the experimental sides versus control sides in the second, third, and fourth week of follow-up periods (p ≤ 0.05). Histologically, on the compression side, the CGF group showed bone resorption and periodontal ligament active reactions from the first week and continued throughout the next three weeks. Also, on the tension side, the CGF group depicted cementoblastic and osteoblastic activities from the first week followed by fibroblastic activities from the second week and all activities continued till the fourth week.
Conclusions
CGF has the potential to effectively enhance orthodontic tooth movement without adverse clinical or histological effects.
Similar content being viewed by others
Background
The duration of orthodontic treatment is crucial in determining patient satisfaction, and there seems to be a growing demand for shorter treatment durations. Orthodontic therapy can vary from a few months to several years. However, most thorough treatments typically last approximately 24 months to finish. However, the treatment duration may be extended in cases of severe malocclusion [1, 2].
During lengthy treatments, patients are more likely to experience root resorption, gingival hyperplasia, and white spot lesions. Moreover, prolonged treatment time may lead to incompliance among adolescent patients, making case completion more challenging [3,4,5,6].
Prior research has examined the use of pharmacological substances such as prostaglandin or hormones like parathyroid hormone to accelerate orthodontic tooth movement (OTM) [7, 8]. Nevertheless, these techniques are biochemically based, demanding in terms of preparation and application, and using supplementary hormones can lead to unfavorable systemic consequences [9,10,11]. Physical ways are costly since they require special devices to perform them [12,13,14]. While surgical techniques are safe and effective, they are also invasive and may not be acceptable to most patients [15, 16]. Flapless micro-osteoperforation was recently introduced, yet contradictory findings were found in the literature regarding this method [17,18,19,20].
Growth Factors (GF) are proteins that control the intricate processes involved in wound healing. They are also crucial in angiogenesis, cell migration, proliferation, and synthetic induction of bone cells, all affecting physiological bone remodeling and repair [21]. Numerous growth factors are involved in the repair of bones; however, two significant groups may be distinguished: growth factors produced from bone and growth factors obtained from autologous blood that are released when platelets are activated [22]. To hasten wound healing and repair, adjuvant agents comprising platelet concentrates (PCs), such as platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF), may be utilized [23].
In 2006, Sacco made the initial proposal for the CGF [24]. After platelet-rich plasma and fibrin, this blood extract represents the third generation. CGF is an organic matrix that is rich in fibrin and comprises growth factors, leukocytes, platelets, immunological cells, and CD34 + stem cells. These components play a vital role in the process of regeneration. CGF is a biologically active substance that promotes bone development and tissue healing [25, 26].
Compared to early-generation platelet concentrates like PRF, more growth factors, increased viscosity, increased tensile strength, and increased adhesive strength are all present in CGF [22]. Together with these, it releases other GFs that promote angiogenesis, matrix remodeling, and cell proliferation, including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), brain-derived growth factor (BDGF), platelet-derived growth factor (PDGF), transforming growth factor-β1 (TGF-β1) and β2 (TGF-β2), and insulin-like growth factor (IGF). It increases the vascular endothelial growth factor content and facilitates soft tissue healing since it incorporates many GFs [23, 26].
CGF in orthodontics can potentially aid tooth movement by promoting accelerated healing and tissue regeneration by enhancing the growth of new blood vessels and improving tissue regeneration, which can be beneficial during orthodontic procedures and expedite recovery [27]. Moreover, The multitude of growth factors in CGF may be beneficial in promoting the action of osteoblasts and osteoclasts that are linked to OTM [28, 29].
From this standpoint, this study aimed to assess how a CGF injection affects the rate of orthodontic tooth movement in New Zealand white rabbits.
Methods
The present investigation was conducted in adherence to the protocols established by the Research Ethics Committee of the Faculty of Dentistry, Tanta University, under ethical code #R-ORTH-3-23-4.
Sample size calculation
The smallest sample size was determined using data from a prior study that examined the effects of injecting PRP against PRF in rabbits [30]. Adopting power of 80% (β = 0.20) to detect a standardized effect size in OTM (primary outcome) of 0.8140, and significance level 5% (accepted α error = 0.05), the negligible necessary sample size was considered to be six sides per subgroup (number of groups = 2) (number of time points = 4) (Total sample size = 6 *2 * 4 = 48 sides) [31]. As split mouth is the adopted design. Thus, 24 New Zealand white rabbits (6 per group) were considered the total number. Any specimen loss resulting from processing error will be substituted to uphold the sa`mple size [32].
Group assignment and animal preparation
Twenty-four adult New Zealand white rabbits (with 48 maxillary quadrants), 7–10 months old, and nearly 2–3 kg weight with normal dentition. Rabbits were obtained from Faculty of Agriculture, Alexandria University. They were selected two weeks before the experiment and examined for any general or dental diseases for exclusion. The animals received food and water and were kept in an environment with controlled lighting and temperature throughout the experimental period, according to the declaration of Helsinki [33]. The 48 maxillary quadrants were used equally in the experimental and control sides and then subdivided according to the follow-up periods into four sub-groups, as presented in Table 1. The surgery was managed under general anesthesia, where 0. 4 ml/Kg of atropine sulfate was injected intramuscularly as a premedication drug, followed by an injection of a mixture of ketamine hydrochloride 10% (ketamine alfasan10%, Woerden, The Netherlands) and xylazine 2% (Adwia, 10th of Ramadan City, Egypt). At a dose of 0.5,0.2 ml/Kg body weight, respectively,
CGF preparation and injection
Ten milliliters of rabbit blood were extracted, and tubes were stored for the one-step centrifugation procedure (NEUATION IFUGE D06) [34] (Fig. 1a): 30 s for acceleration, 2 min for 2700 rpm, 4 min for 2400 rpm, 3 min for 3000 rpm, and 36 s for deceleration and stop. This leads to the following four phases [35]: (Fig. 1b).
-
1.
The superior phase is symbolized by serum.
-
2.
A fibrin buffy coat represents the transitional stage.
-
3.
Growth factors represent the liquid phase.
-
4.
The lower phase is represented by red blood cells.
Utilizing an insulin syringe, seventy units of the liquid phase was administered submucosally into the buccal and palatal regions across from the maxillary first molars in the same manner that the local anesthetic injection was done on all experimental sides [36].
Orthodontic procedures
A simple orthodontic appliance was installed on each maxillary quadrant using a NiTi closed coil spring (9 mm, DynaFlex, USA, www.dynaflex.com). The spring is attached from each side to the ligature wire. These ligature wires were then ligated to holes generated using rosehead burs in the upper first molars and the cervical portion of central incisors. The force-gauge measurement indicated that the spring stretched to deliver 40 cN force [37]. The ends of the ligature wires were covered by flowable composite to prevent gingival irritation. The springs were not reactivated between the follow-up periods.
A digital caliper (Vernier Digital Caliper, Future Electronics, Egypt) was used to measure how far the first molars were mesialized on both the experimental and control sides by measuring the distance between two reference points: the mesial contact point of the first molars to the distal contact point of the central incisors. All measurements were done by the same investigator who was blinded to the treatment groups.
Animal’s euthanization
Six rabbits were euthanized after 1, 2, 3, and 4 weeks, respectively. The rabbits authenization was done by intramuscular injection of overdose of xylazine HCl (30 mg/kg; Adwia, 10th of Ramadan City, Egypt) and ketamine HCl (70 mg/kg; ketamine alfasan10%, Woerden, The Netherlands) and scarified then dissected.
Histologic evaluation
The maxilla of each rabbit was dissected into right and left halves, coded, and fixed into 10% calcium formol (Aqua Med Company, Egypt) for 48 h. After that, they were washed and decalcified through immersion in EDTA 10%. Then, the samples underwent progressively higher alcohol concentrations for dehydration before being conventionally embedded in paraffin. Hematoxylin and eosin (H&E) were used to stain six µm thick sagittal serial sections for histological analysis under a light microscope (Leica ICC50 HD) outfitted with a digital camera, and images of representative regions were captured and annotated [38]. PDL, alveolar bone, and the root of the upper first molars were histologically observed from the mesial (compression side) and distal (tension side). Histological analysis was done by a single investigator in a blinded manner.
Statistical analysis
All data were statistically analyzed using version 20.0 of the IBM SPSS software package (Armonk, NY: IBM Corp). The Shapiro-Wilk test was used to check for normality in continuous data. Distributed data were represented by mean, standard deviation, range, and median. A paired t-test was employed to compare two periods for normally distributed quantitative variables. A one-way ANOVA test was performed to compare the different studied periods, and a post hoc Tukey test was performed for pairwise comparisons. The obtained results were declared significant at the 5% level. All data were labelled with numbers and the statistician was also blinded regarding the study groups.
Results
All animals survived the experimental procedures and the subsequent follow-up phase. No inflammation-related symptoms were noticed. Only 20% of the animals experienced gingival edema related to coil springs, which eventually resolved within a few days.
Tooth movement rate
The distance measured between the first molars and incisors was decreased throughout follow-ups in both the experimental and control sides. However, the CGF results in a higher rate of tooth movement starting from the second to the fourth weeks compared to the control sides (3.72 ± 0.30 vs. 2.49 ± 0.53, 4.81 ± 0.56 vs. 3.23 ± 0.35, and 6.56 ± 0.49 vs. 4.72 ± 0.24, respectively). According to the Paired t-test results, a significant difference between the experimental and control sides was demonstrated in all examined periods (p ≤ 0.05) except for the first week (p = 0.951). (Table 2; Fig. 2)
There was a significant difference between all follow-up weeks, according to the One-Way ANOVA test and the post-hoc test (Tukey) used for comparing the various studied periods of each group. (Table 3)
Histologic findings
At the compression side
In the control group, the PDL appeared compressed after one week of orthodontic force application, followed by PDL hyalinization in different areas and fibroblastic activity after two weeks of initial orthodontic application (Fig. 3C1,3). Moreover, in week 3, the PDL increased hyalinization and bone resorption, and irregularly arranged osteocytes in the woven bone appeared (Fig. 3C5). PDL showed the same changes at four weeks as the previous week, with three plus cementoblastic and fibroblastic activities (Fig. 3C7).
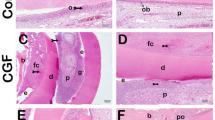
Photomicrographs of the compressive side of both groups. (C1& C2) after one week in control and CGF groups, respectively, showing compressed PDL. C2, the CGF group, shows the initiation of bone resorption (arrowhead) at the bone surface. (C3) control group after two weeks showing PDL fibers with obvious hyalinization (H) and large active fibroblasts (red arrows). (C4) CGF group after two weeks showing PDL fibers with dilated blood vessels (V), active cementoblasts (yellow arrows), and areas of bone resorption (arrowheads) at the bone surface. Also, a large area of new bone deposition (star) is delineated from the old one with dense appositional lines (curved arrows). (C5) The control group showed PDL fibers with obvious hyalinization (H) and areas of bone resorption (arrowheads) at the bone surface at three weeks. The new woven bone (stars) is delineated from the old one with dense appositional lines (curved arrows) with irregularly arranged osteocytes (green arrows). (C6) CGF group at three weeks showing PDL fibers with noticeable large active fibroblasts (red arrows), active cementoblasts (yellow arrows), and multiple areas of bone resorption (arrowheads) at the bone surface. Also, areas of new bone deposition (stars) are delineated from old bone with appositional lines (curved arrows), and large multiple areas of extravasated blood in the bone (E) could be detected. (C7) The control group, after four weeks, showed PDL fibers with noticeable large active fibroblasts (red arrows), cementoblastic activity (yellow arrows), and multiple areas of bone resorption (arrowheads) at the bone surface. The woven bone fills the socket (AB) with irregularly arranged osteocytes (green arrow). (C8) CGF group after four weeks of orthodontic force application showing PDL fibers with noticeable large active fibroblasts (red arrows), active cementoblasts (yellow arrows), and multiple areas of bone resorption (arrowheads) at the bone surface. The woven bone fills the socket (AB). It shows irregularly arranged osteocytes (green arrow), an area of new bone deposition (star) that is delineated from the old one with dark lines (curved arrows), and large multiple areas of extravasated blood (E). Notice the long arrow that indicates the direction of the tooth movement at the bottom of each figure. (H&E stain, C1, 2, 3, 4, 5, 6, 7, 8 × 100)
In the CGF group, the PDL appeared compressed after one week of initial orthodontic force application, plus the initiation of bone resorption at the bone surface was depicted (Fig. 3C2). After two weeks of orthodontic treatment, PDL fibers showed dilated Blood vessels, active cementoblasts, and areas of bone resorption at the bone surface (Fig. 3C4). The PDL showed the same histological changes as the previous interval plus noticeable large active fibroblasts and irregularly arranged large osteocytes in the woven bone that filled the socket in weeks 3 and 4 (Fig. 3C6, 8)—compared with the control group, principal fibers of PDL and fibroblasts displayed a more regular pattern of organization devoid of inflammatory infiltrates or hyaline zones.
At the tension side
In the control group, The PDL appeared tense, and some small osteocytes appeared in the bone of the socket after one week of orthodontic force application (Fig. 4T1). At 2-week intervals, the tensed PDL showed cementoblastic activity and irregularly arranged large osteocytes occupying the woven bone of the socket (Fig. 4T3). Besides the irregularly arranged large osteocytes in the woven bone and the dilated blood vessels, areas of hyalinization appeared after 3-week intervals (Fig. 4T5). Also, at 4-week intervals, the PDL of the control group showed cementoblastic activity and woven bone osteoblastic activity (Fig. 4T7).
Photomicrographs of the tension side of both groups. (T1) The control group, after one week, showed tensed PDL and bone filling the socket (AB) with a small number of large osteocytes (green arrow). (T2) CGF group after one week showing tensed PDL, cementoblastic activity (yellow arrows), and the woven bone filling the socket (AB) with irregularly arranged large osteocytes (green arrow) as well as extravasated red blood cells (E) scattered throughout the alveolar bone. (T3) Control group at two weeks showing cementoblastic activity (yellow arrows), irregularly arranged large osteocytes (green arrow) occupying the newly formed woven bone of the socket (star) that delineated from the old one with dark lines (curved arrows). Also, extravasated red blood cells (E) at random areas of the bone could be detected. (T4) CGF group after two weeks showing cementoblastic activity (yellow arrows) The woven bone is filling the socket (AB) with irregularly arranged large osteocytes (green arrow) and active osteoblasts (black arrows). Also, extravasated red blood cells (E) scattered throughout the alveolar bone could be noticed. (T5) The control group showed PDL fibers after three weeks, with areas of hyalinization (H) and dilated blood vessels (V). The woven bone fills the socket (AB) with irregularly arranged large osteocytes (green arrow). (T6) CGF group after four weeks showing PDL fibers with dilated blood vessels (V), large active fibroblasts (red arrows), and cementoblastic activity (yellow arrows). The woven bone fills the socket (AB) with new bone formation (stars) separated from old bone with appositional lines (curved arrows). It contains irregularly arranged osteocytes (green arrow) and extravasated blood (E) areas. Notice the large cementocytes inside the root cementum (white arrows). (T7) The control group showed PDL fibers with cementoblastic activity at four weeks (yellow arrows). The woven bone fills the socket (AB) with irregularly arranged large osteocytes (green arrow) and osteoblastic activity (black arrows). Notice the extravasated blood (E) areas with inflammatory infiltrates (branched arrow). (T8) CGF group after three weeks showing PDL fibers with dilated Blood vessels (V), large active fibroblasts (red arrows), and cementoblastic activity (yellow arrows). The woven bone fills the socket (AB) with an area of newly formed bone (star) that is delineated from old bone with appositional lines (curved arrows), irregularly arranged large osteocytes (green arrow), extravasated blood vessels (E), and osteoblastic activity (black arrows). Notice the long arrow that indicates the direction of the tooth movement at the bottom of each figure. (H&E stain, T1, 2, 3, 4, 5, 6, 7, 8 × 100)
In the CGF group, The PDL appeared tensed, and cementoblastic activity was detected. Also, the woven bone with irregularly arranged large osteocytes was noticed after one week of orthodontic force application (Fig. 4T2). At a 2-week interval, the PDL and the bone showed the same changes as the previous interval, plus cementoblastic and osteoblastic activities (Fig. 4T4). At 3-week intervals, the PDL and the woven bone showed the same changes as the previous intervals, plus dilated blood vessels and fibroblastic and osteoblastic activities were detected (Fig. 4T6). At 4-week intervals, the PDL and the woven bone showed the same changes as the previous intervals, plus large cementocytes inside the root cementum were detected (Fig. 4T8). Different regions of the alveolar bone showed extravasated blood vessels compared to the control group.
Discussion
Based on previous literature, this is the first trial assessing the utilization of CGF injection for accelerating orthodontic tooth movement.
New Zealand white rabbits were selected as an animal model for this study as they are considered one of the most suitable animals for getting a clear image of bone alterations under stress [39]. Furthermore, the bone turnover in the rabbits is rapid more than in other species [40].
CGF contains many growth factors; hence, it has been proven that they are key elements for bone cell recruitment, activation, proliferation, differentiation, and survival. CGF was utilized in this experimental study [25, 41, 42].
In this investigation, CGF was injected submucosally buccal and palatal opposite the maxillary first molars on the experimental sides. Submucosal application is the predominant approach for administering local pharmacological agents to accelerate tooth movement [7, 43]. The injection technique and amount were similar to the previous study, which utilized PRP for the acceleration of OTM [36].
NiTi coil springs were chosen because they do not display quick force decay, produce a steady light force that has been testified to be beneficial in space closing, and give superior oral health compared to elastomeric chains [44, 45].
During the first week, nonsignificant differences were observed between the two groups. This phenomenon might likely be attributed to the earliest stage of orthodontic treatment, which occurs within the PDL space and may persist for approximately one week before any more tooth movement becomes noticeable [46]. Conversely, a significant increase in tooth movement was observed during the second, third, and fourth weeks following CGF injection, compared to the control sides (p = 0.003, 0.001, 0.002; respectively). These results indicate a clear association between CGF injection and the hastening of orthodontic tooth movement. This was following the results obtained by previous animal studies utilizing PRP injection [30, 47].
The study found that the rate of tooth movement increased by approximately 1.5 times after the injection of CGF, compared to the control group. The acceleration seen in this study was comparable to the acceleration achieved after PRP injection as reported by Alaa et al. [30], and after the administration of moderate and high concentration PRP as reported by Gulec et al. [37]. In contrast, Rashid et al. [47] observed a 2.13-fold increase in acceleration on the PRP side, which is greater than the findings of this investigation. The possible cause of this could be attributed to variations in their injection method (intraligamental and submucosal) and the higher frequency of injections employed in their research. Histologically, the rate of tooth movement can be controlled by bone remodeling, bone formation at the tension side, and bone resorption at the compression side during tooth movement [48]. Our results were consistent with Choi et al. [49], who found that bone remodeling in rats jumps after the first week, with the activity continuing till the start of the third week. Moreover, these outcomes agree with those of Pavlin et al. [50], where there was evidence of new bone formation from the first week to day 12. The appearance of irregularly arranged large osteocytes occupying the woven bone of the socket at the tension side of both groups appeared earlier in the CGF group. This could be clarified by the ability of the CGF to enhance osteogenic differentiation and mineralization of the cranial defect model of rats in vivo [50]. Also, bone resorption on the compression side appeared earlier in the CGF group than in the control group. The increased rate of bone resorption could have been explained by the existence of CGF several growth factors that induced osteoblastic and osteoclastic activity [41]. On the compression side, the clinical finding is verified. The PDL fibers were arranged irregularly in the two groups but to a lesser extent in the CGF group. This was mainly the result of the compression effect from the tooth to the PDL, which would finally followed by tooth movement through a complex process of molecular and cellular routes [51]. Moreover, more widened blood vessels were observed in PDL in the CGF group than in the control group. This was most likely brought on by the inflammatory mediators that the PDL tissues released secondary to the mechanical effect of orthodontic treatment and those initially present within the CGF as vascular endothelial growth factor (VEGF), a potent vascular dilator [52, 53].
Study limitations and clinical significances
Our study had some limitations; the long-term evaluation, the efficacy of different doses, and injection frequency are critical and may have a significant impact on clinical implementation of this new technique. Second, due to anatomical differences, the influence on mandibular teeth must be explored in future investigations. However, under the current study settings, the results could be clinically significant for orthodontic practice. CGF could be utilized as a simple, safe, and cost-effective method for accelerating orthodontic tooth movement.
Conclusion
Within the limitation of this study, it could be concluded that CGF has the potential to effectively enhance orthodontic tooth movement without adverse clinical or histological effects.
Data availability
On reasonable request, the datasets utilized and analyzed during the present study are accessible from the corresponding author.
Abbreviations
- BDGF:
-
Brain-derived growth factor
- CGF:
-
Concentrated Growth Factor
- FGF:
-
Fibroblast growth factor
- H&E:
-
Hematoxylin and eosin
- IGF:
-
Insulin-like growth factor
- OTM:
-
Orthodontic tooth movement
- PCs:
-
Platelet concentrates
- PDGF:
-
Platelet-derived growth factor
- PRF:
-
Platelet-rich fibrin
- PRP:
-
Platelet-rich plasma
- TGF-β1:
-
Transforming growth factor-β1
- TGF-β2:
-
Transforming growth factor-β2
- VEGF:
-
Vascular endothelial growth factor
References
Mavreas D, Athanasiou AE. Factors affecting the duration of orthodontic treatment: a systematic review. Eur J Orthod. 2008;30(4):386–95.
Riedmann T, Georg T, Berg R. Adult patients’ view of orthodontic treatment outcome compared to professional assessments. J Orofac Orthop. 1999;60(5):308–20.
Younis O, Hughes DO, Weber FN. Enamel decalcification in orthodontic treatment. Am J Orthod. 1979;75(6):678–81.
Sadowsky C, BeGole EA. Long-term effects of orthodontic treatment on periodontal health. Am J Orthod. 1981;80(2):156–72.
Rönnerman A, Thilander B, Heyden G. Gingival tissue reactions to orthodontic closure of extraction sites. Histologic and histochemical studies. Am J Orthod. 1980;77(6):620–5.
Southard KA, Tolley EA, Arheart KL, Hackett-Renner CA, Southard TE. Application of the Millon adolescent personality inventory in evaluating orthodontic compliance. Am J Orthod Dentofac Orthop. 1991;100(6):553–61.
Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod. 1984;85(6):508–18.
Li F, Li G, Hu H, Liu R, Chen J, Zou S. Effect of parathyroid hormone on experimental tooth movement in rats. Am J Orthod Dentofac Orthop. 2013;144(4):523–32.
Soma S, Iwamoto M, Higuchi Y, Kurisu K. Effects of continuous infusion of PTH on experimental tooth movement in rats. J Bone Min Res. 1999;14(4):546–54.
Kawasaki K, Shimizu N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med. 2000;26(3):282–91.
Huang H, Williams RC, Kyrkanides S. Accelerated orthodontic tooth movement: molecular mechanisms. Am J Orthod Dentofac Orthop. 2014;146(5):620–32.
Cruz DR, Kohara EK, Ribeiro MS, Wetter NU. Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: a preliminary study. Lasers Surg Med. 2004;35(2):117–20.
Kau CH, Nguyen JT, English J. The clinical evaluation of a novel cyclical force generating device in orthodontics. Orthod Pract US. 2010;1(1):10–5.
Xue H, Zheng J, Cui Z, Bai X, Li G, Zhang C, et al. Low-intensity pulsed ultrasound accelerates tooth movement via activation of the BMP-2 signaling pathway. PLoS ONE. 2013;8(7):e68926.
Long H, Pyakurel U, Wang Y, Liao L, Zhou Y, Lai W. Interventions for accelerating orthodontic tooth movement: a systematic review. Angle Orthod. 2013;83(1):164–71.
Dibart S, Sebaoun JD, Surmenian J. Piezocision: a minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Compend Contin Educ Dent. 2009;30(6):342.
Alikhani M, Raptis M, Zoldan B, Sangsuwon C, Lee YB, Alyami B, et al. Effect of micro-osteoperforations on the rate of tooth movement. Am J Orthod Dentofac Orthop. 2013;144(5):639–48.
Alkebsi A, Al-Maaitah E, Al-Shorman H, Abu Alhaija E. Three-dimensional assessment of the effect of micro-osteoperforations on the rate of tooth movement during canine retraction in adults with class II malocclusion: a randomized controlled clinical trial. Am J Orthod Dentofac Orthop. 2018;153(6):771–85.
Aboalnaga AA, Salah Fayed MM, El-Ashmawi NA, Soliman SA. Effect of micro-osteoperforation on the rate of canine retraction: a split-mouth randomized controlled trial. Prog Orthod. 2019;20(1):21.
Alfawal AM, Hajeer MY, Ajaj MA, Hamadah O, Brad B. Effectiveness of minimally invasive surgical procedures in the acceleration of tooth movement: a systematic review and meta-analysis. Prog Orthod. 2016;17(1):33.
McGuire MK, Scheyer ET, Schupbach P. Growth factor-mediated treatment of recession defects: a randomized controlled trial and histologic and microcomputed tomography examination. J Periodontol. 2009;80(4):550–64.
Civinini R, Macera A, Nistri L, Redl B, Innocenti M. The use of autologous blood-derived growth factors in bone regeneration. Clin Cases Min Bone Metab. 2011;8(1):25–31.
Ramakrishnan T, Shobana P, Sekhar V, Nirmala J, Ebenezer SK. Concentrated growth factor membrane-a novel barrier for accelerated repair of gingival defect along with sliding flap technique. Int J Curr Res Rev. 2016;8:1–5.
Sacco L, Lecture. International academy of implant prosthesis and osteoconnection. Lecture. 2006;12:4.
Nityasri, Aromal S, Pradeep KY, Kalaivani V, Raja P. Role of CGF (concentrated growth factor) in periodontal regeneration. J Dent Health Oral Disord Ther. 2018;9(5):350–2.
Kshirsagar JT, Rubine S. Innovation in regeneration–concentrated growth factor. Int J Appl Dent Sci. 2017;3(2):206–8.
Farshidfar N, Amiri MA, Firoozi P, Hamedani S, Ajami S, Tayebi L. The adjunctive effect of autologous platelet concentrates on orthodontic tooth movement: a systematic review and meta-analysis of current randomized controlled trials. Int Orthod. 2022;20(1):100596.
Kumar AA, Saravanan K, Kohila K, Kumar SS. Biomarkers in orthodontic tooth movement. J Pharm Bioallied Sci. 2015;7(Suppl 2):S325–30.
Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–70.
Alaa S, Fouda AM, Grawish ME, Abdelnaby YL. The effect of submucosal injection of platelet-rich fibrin vs. platelet-rich plasma on orthodontic tooth movement in rabbits; 28 days follow-up. Int Orthod. 2023;21(1):100715.
Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35(2):121–6.
Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126(2):619–25.
Ashall V, Morton D, Clutton E. A declaration of Helsinki for animals. Vet Anaesth Analg. 2023;50(4):309–14.
Ellithy AA, El-Hessy AA, El-Zefzaf EA. Concentrated growth factors Versus platelet Rich Fibrin in the treatment of Intrabony defects in chronic Periodontitis. Int J Dent Ora Hea. 2021;7:60–75.
Nityasri AS, Pradeep KY, Kalaivani V, Raja P. Role of CGF (concentrated growth factor) in periodontal regeneration. J Dent Health Oral Disord Ther. 2018;9(2):350–2.
Alawy SB, Gaballah SM, Ellaithy MM. Effect of submucosal platelet Rich plasma injection on the Rate of Orthodontic Tooth Movement: a Randomized Split Mouth Trial. Int J Dent Oral Health. 2022;8(1):1–7.
Güleç A, Bakkalbaşı B, Cumbul A, Uslu Ü, Alev B, Yarat A. Effects of local platelet-rich plasma injection on the rate of orthodontic tooth movement in a rat model: a histomorphometric study. Am J Orthod Dentofac Orthop. 2017;151(1):92–104.
Bancroft JD, Gamble M. Theory and practice of histological techniques. 5th ed. London: Churchill Livingstone; 2002.
Storey E. Tissue response to the movement of bones. Am J Orthod. 1973;64(3):229–47.
Castañeda S, Largo R, Calvo E, Rodríguez-Salvanés F, Marcos ME, Díaz-Curiel M, et al. Bone mineral measurements of subchondral and trabecular bone in healthy and osteoporotic rabbits. Skeletal Radiol. 2006;35(1):34–41.
Yu B, Wang Z. Effect of concentrated growth factors on beagle periodontal ligament stem cells in vitro. Mol Med Rep. 2014;9(1):235–42.
Andrade I Jr., Sousa AB, da Silva GG. New therapeutic modalities to modulate orthodontic tooth movement. Dent Press J Orthod. 2014;19(6):123–33.
Seifi M, Hamedi R, Khavandegar Z. The effect of thyroid hormone, prostaglandin E2, and calcium gluconate on Orthodontic Tooth Movement and Root Resorption in rats. J Dent (Shiraz). 2015;16(1 Suppl):35–42.
Dixon V, Read MJ, O’Brien KD, Worthington HV, Mandall NA. A randomized clinical trial to compare three methods of orthodontic space closure. J Orthod. 2002;29(1):31–6.
Samuels RH, Rudge SJ, Mair LH. A comparison of the rate of space closure using a nickel-titanium spring and an elastic module: a clinical study. Am J Orthod Dentofac Orthop. 1993;103(5):464–7.
Reitan K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am J Orthod. 1967;53(10):721–45.
Rashid A, ElSharaby FA, Nassef EM, Mehanni S, Mostafa YA. Effect of platelet-rich plasma on orthodontic tooth movement in dogs. Orthod Craniofac Res. 2017;20(2):102–10.
Pedraza JLM, Marquezan M, Nojima LI, Nojima MDC. Macroscopic and microscopic evaluation of flapless alveolar perforations on experimental tooth movement. Dent Press J Orthod. 2018;23(6):73–9.
Choi J, Baek SH, Lee JI, Chang YI. Effects of clodronate on early alveolar bone remodeling and root resorption related to orthodontic forces: a histomorphometric analysis. Am J Orthod Dentofac Orthop. 2010;138(5):548. .e1-8; discussion -9.
Pavlin D, Goldman ES, Gluhak-Heinrich J, Magness M, Zadro R. Orthodontically stressed periodontium of transgenic mouse as a model for studying mechanical response in bone: the effect on the number of osteoblasts. Clin Orthod Res. 2000;3(3):55–66.
Dong K, Zhou WJ, Liu ZH, Hao PJ. The extract of concentrated growth factor enhances osteogenic activity of osteoblast through PI3K/AKT pathway and promotes bone regeneration in vivo. Int J Implant Dent. 2021;7(1):70.
Andrade I, Taddei SRA, Souza PEA. Inflammation and tooth Movement: the role of cytokines, chemokines, and growth factors. Semin Orthod. 2012;18(4):257–69.
Stanca E, Calabriso N, Giannotti L, Nitti P, Damiano F, Stanca BDC, et al. Analysis of CGF Biomolecules, structure and Cell Population: characterization of the stemness features of CGF cells and osteogenic potential. Int J Mol Sci. 2021;22(16):8867.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.B.A (A). Study conception and design, installation of orthodontic appliance, data collection, sharing in writing, and revising the manuscript. M.A.E (B) Study conception and design, blood centrifugation and CGf preparation and injection, data collection, sharing in writing, and revising the manuscript. E.M.S. (c) Responsible for study conception and design. Participated in performing all required data analysis, shooting and Revising histological results and analysis, editing and reviewing the final manuscript. W.Y.E.(D) Study conception and design, histological analysis, data collection, shared in writing and revising the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The ethical committee (REC) of the Faculty of Dentistry, Tanta University, approved the study under the code (#R-ORTH-3-23-4).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alawy, S.B., El Meligy, M.A.A., Salem, E.M. et al. Impact of concentrated growth factor (CGF) injection on acceleration of orthodontic tooth movement in rabbits. BMC Oral Health 24, 783 (2024). https://doi.org/10.1186/s12903-024-04520-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04520-2