Abstract
Background
Current research has been inconclusive regarding whether hepatitis B infection is associated with an increased risk of periodontitis. This study aims to test the null hypothesis that no association exists between hepatitis B infection and an increased risk of periodontitis using the National Health and Nutrition Examination Survey (2009–2014).
Methods
We performed a cross-sectional study using the National Health and Nutrition Examination Survey (NHANES) database (2009–2014) to assess the rate of the prevalence of periodontitis in patients with and without hepatitis B infection. Participants who had tested for hepatitis B and periodontitis were included. The included participants were divided into no/mild periodontitis and moderate/severe periodontitis groups according to their periodontal status. The association between hepatitis B infection and chronic periodontitis was evaluated by multivariable regression analyses adjusting for age, gender, race/ethnicity, education level, income-to-poverty ratio, smoking, alcohol, BMI, ALT, AST, creatinine, hypertension, and diabetes.
Results
A total of 5957 participants were included and divided into two groups: inactive periodontitis group (n = 3444) and active periodontitis group (n = 2513). The results showed that participants with hepatitis B had a higher risk of periodontitis. After adjusting for covariables, adults with hepatitis B infection were 38% more likely to have periodontitis compared to those without hepatitis B infection (95% Confidence Interval [CI]:1.085–1.754).
Conclusions
In general, the results suggest that CHB is positively associated with the more severe periodontitis. These results suggest that people with hepatitis B infection should take good periodontal care measures to avoid the occurrence and development of periodontitis.
Similar content being viewed by others
Introduction
The burden of periodontitis continues to be a global public health problem, with the majority of people with periodontitis aged between 55 and 59 years; however, the prevalence of periodontitis is increasing in younger people [1]. There are more than 700 types of bacteria in the oral cavity, and these bacteria usually co-exist in a harmonious state, called probiotics, when symbiotic bacteria do not allow harmful bacteria to cause disease [2]. These bacteria can also be found in the gingival groove or sulcus, the narrow space that is delimited by the tooth’s surface and the gingiva [3]. If the gingival sulcus is not cleaned properly and regularly through professional and at-home methods, this can lead to the emergence of highly pathogenic bacteria. Therefore, this subgingival pathogenic biofilm causes periodontal inflammation (periodontitis) [4]. Periodontitis is a chronic inflammation that damages the supporting tissues of the teeth and, if the condition continues to develop, can lead to loss of alveolar bone, loosening of teeth, and eventually tooth loss [5]. In the past few decades, a large number of studies have shown that the consequences of periodontitis are not only the destruction of normal tooth function and structure [6]. Periodontal tissue is connected to the rest of the body by blood and lymph [7]. Therefore, every pathological change that can disrupt general homeostasis has the potential to affect periodontal health. At the same time, periodontitis can also affect the overall health of patients, as well as the occurrence and development of specific diseases [8]. Researchers studied the bi-directional relationship between periodontitis and systemic health and disease, resulting in the concept of “periodontal medicine” [9]. More and more literature studies show that periodontitis is associated with many systemic diseases, such as diabetes, Alzheimer’s disease, and respiratory infection. The biological mechanism underlying this correlation still needs further study [10].
The oral cavity is a dynamic open system with various ecological niches in various forms and conditions, which is a suitable environment for microbial colonization [11, 12]. In addition to being colonized by bacteria, archaea, and fungi, the mouth can harbor a variety of viruses. Cytomegalovirus, Epstein-Barr virus, human herpesvirus 6, HIV, and herpes simplex virus type 1, as well as hepatitis A, B, and C, anelloviruses (such as torque teno virus), and papillomaviruses, can be detected in saliva and gingival tissues [13]. In a recent meta-analysis, several of these viruses were found to be associated with periodontitis [14], possibly through direct damage, coordination with the local bacterial community, and/or regulation of the immune response [15].
Hepatitis B is an infection caused by hepatitis B virus that damages the liver [16]. The virus is mainly transmitted through contact with infected blood or bodily fluids [17]. The infection enters a chronic phase, which can lead to life-threatening complications such as cirrhosis and liver cancer [18]. A meta-analysis showed that there is sufficient evidence in the existing literature to support the effect of HBV infection on the oral environment [19]. Studies on the effects of hepatitis B virus infection on the oral cavity mainly involve the detection of viral antigens in saliva and gingival crevicular fluid and rarely involve the clinical, dental, or periodontal conditions of patients [19].
Furthermore, previous research has shown a correlation between periodontitis and liver disease [20, 21]. A retrospective study from Japan suggests that periodontitis may be associated with the progression of viral liver disease [20]. Impaired oral health in patients with hepatitis C infection can be the result of liver dysfunction, compromised immune systems, or a lack of motivation for infected patients to seek dental care [22, 23]. A study utilizing Escherichia coli lipopolysaccharide and Streptomyces griseus proteases to induce periodontitis and steatosis in an animal model demonstrated this relationship [24]. Additionally, Porphyromonas gingivalis, a significant periodontal pathogen, has been found in the livers of patients with hepatic fibrosis, impacting the progression of liver disease [25].
Although the relationship between periodontitis and other liver diseases has been analyzed and evaluated from different perspectives [20,21,22,23,24,25], the relationship between periodontitis and hepatitis B needs to be further studied. It is valuable to study the relationship between HBV infection and periodontitis in a large representative population. Therefore, we analyzed secondary data based on available data from the National Health and Nutrition Examination Survey (NHANES). The study aims to determine whether there was a significant relationship between hepatitis B infection and periodontitis and to understand the associated confounding factors. Therefore, this study tests the null hypothesis that there is no difference in the prevalence of periodontitis between individuals with and without hepatitis B infection.
Materials and methods
Study design and data source
The NHANES is a continuous survey research that provides population estimates related to the nutrition and health of adults and children in America. The survey employs a stratified, multistage probability design to recruit a representative sample of the American population. Data were obtained via personal structured interviews at home, health examinations at a mobile examination center, and specimen analyses in the laboratory. NHANES 2009–2014 was approved by The National Center for Health Statistics (NCHS) Ethics Review Board and conducted following the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from all participants. The acquisition and analysis of data was consistent with NHANES research requirements.
In this cross-sectional retrospective study, we selected NHANES data from the 2009–2010, 2011–2012, and 2013–2014 cycles. The Centers for Disease Control and Prevention (CDC) manages the National Center for Health Statistics (NCHS), which conducts NHANES assessments of the health and nutrition status of children, adults, and older adults. All NHANES protocols were approved by the National Center for Health Statistics Ethics Review Committee with written informed consent from all participants [26]. This modeling investigation was exempt from review because it used published de-identified data sets that included no personally identifiable information. The data from the CDC website can be downloaded for free (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
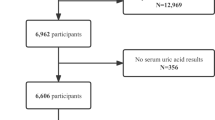
The Inclusion and exclusion procession is shown in Fig. 1. We used the data from the NHANES 2009–2014 cycles. 5957 patients with clear hepatitis B, periodontitis, and other covariable data were included to explore the association between chronic hepatitis B infection and the risk of periodontitis. Among them, 419 had hepatitis B infection, while 5,538 had never had hepatitis B infection.
Assessment of periodontitis
The dependent variable was periodontitis (dichotomous variable). A mobile examination center (MEC) was used for periodontal examination of participants aged 30 years and older. For oral health examinations, the NHANES operating manual describes the training and calibration process [27]. According to the 2012 CDC/ American Association of Periodontitis (AAP) case definition for periodontitis, participants must have at least two teeth that meet specific detection thresholds, and there are four grades of periodontitis: no, mild, moderate, and severe [28]. Mild periodontitis was defined as ≥ 2 interproximal sites with AL ≥ 3 mm and ≥ 2 interproximal sites with PD ≥ 4 mm (not on the same tooth) or one site with PD ≥ 5 mm. Moderate periodontitis was defined as ≥ 2 interproximal sites with AL ≥ 4 mm (not on the same tooth), or ≥ 2 interproximal sites with PD ≥ 5 mm (not on the same tooth). Severe periodontitis was defined as ≥ 2 interproximal sites with AL ≥ 6 mm (not on the same tooth) and ≥ 1 interproximal site with PD ≥ 5 mm. This study defines people with no and mild periodontitis as inactive periodontitis, and people with moderate and severe periodontitis as active periodontitis.
HBV serological testing
In this study, Anti-HBc was used as a key indicator because hepatitis B surface antigen positive indicates that the patient is currently infected with acute or chronic hepatitis, but does not include clinically cured cases [29]. However, HBV core antibodies remain positive after the acute phase of inflammation has resolved, so the selection of HBV core antibody-positive patients can include all patients with current or previous hepatitis B infection [30]. Hepatitis B core antibodies are tested by using the VITROS Anti-HBc test. The VITROS Anti-HBc assay is performed using the VITROS Anti-HBc Reagent Pack and VITROS Immunodiagnostic Products Anti-HBc Calibrator on the VITROS ECi/EciQ Immunodiagnostic System.
Assessment of covariates
In the demographic data in the NHANES database, we collected relevant information about the respondents’ age, gender, race, income level, education, etc. Ethnicity is divided into Mexican Americans, other Hispanics, non-Hispanic whites, non-Hispanic blacks, and others. The family income level is divided into low-income, middle-income, and high-income. The level of education is classified as below high school, high school or equivalent, university or above, and others.
The examination information contains the physical examination data of the respondents. In this column, we collected data on the body mass index (BMI) of the respondents. BMI is calculated based on a person’s weight and height(<18.5 kg/m2,18.5–24.9 kg/m2, 25.0–29.9 kg/m2, > = 30.0 kg/m2) [31].
In the Laboratory test data, the correlation results of the respondent tests are recorded. Here, we collected data on hepatitis B core antibodies (anti-HBc). In addition, data were collected for relevant covariates ALT, AST, and creatinine that may have influenced the results.
Finally, we collected data about smoking, alcohol, hypertension, and the presence of diabetes in the respondents in the questionnaire column. Smoking status was categorized as “nonsmokers” (lifetime use of < 100cigarettes), “former smokers” (previous history of smoking but no longer a smoker at the time of interview), or “current smokers” (lifetime use of ≥ 100 cigarettes and who currently smoke cigarettes) [32]. According to the average number of alcoholic drinks consumed per week in the past 12 months, alcohol consumption was categorized into three strata (0–1, 1-< 8, and ≥ 8 drinks per week) and defined as nonuse, moderate use, and heavy use, respectively. A “drink” was defined as a 12-ounce beer, a 5-ounce glass of wine, or one-and-half ounces of liquor [33].
Statistical analyses
According to the hepatitis indicators, the respondents who were positive and negative for hepatitis were divided into two different groups. In terms of data statistics, the data of categorical variables are expressed as numbers with percentages (N%), while the data of continuous variables are expressed as mean values with standard deviations (mean ± SD). We used Chi-square tests to compare the percentages of categorical variables between the different groups. Multivariable Logistic regressions were used to examine the association between hepatitis B infection and periodontitis. We used the null hypothesis that there was no relationship between hepatitis B infection and periodontitis. The crude model does not adjust for covariates, Model I adjusts for sociodemographic data, and Model II adjusts for all covariates. Odds ratios (ORs) were calculated with 95% confidence intervals (CIs) from multivariate logistic regression analyses were reported to demonstrate the observed associations. At the same time, we stratified the relationship between hepatitis B infection and periodontitis by gender and BMI to assess whether there is a potential impact change between hepatitis B infection and periodontitis. All the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA). All tests were two-sided and P values lower than 0.05 were considered statistically significant, and the null hypothesis was rejected.
Results
Description of the study sample
The study sample consisted of 5957 participants from 2009 to 2014. Approximately 7.0% of participants had hepatitis B infection, and 42.18% suffered from periodontitis. The subjects in the group with active periodontitis are more likely to be 55–69 years old, male, non-Hispanic white, with higher BMI, less smoking (less than 100 cigarettes), no hypertension and diabetes, and moderate use of alcohol (p < 0.05) (Table 1). In Table 2, we show the periodontal clinical indicators of hepatitis B-infected and uninfected patients. The results showed that the average Pocket depth and the average degree of attachment loss of hepatitis B infected people were deeper and more serious than that of uninfected people, and more sites with PD ≥ 4 mm and AL ≥ 3 mm. In addition, the proportion of active periodontitis is also higher in the hepatitis B-infected population.
Relationship between hepatitis B infection and periodontitis
The multivariable logistic regression analysis between chronic hepatitis B and periodontitis is shown in Table 3. The positive association between hepatitis B and periodontitis was revealed in all models. Model I only adjusts for sociodemographic data, and the adjusted OR increases relatively (OR 1.390, 95% CI 1.100–1.756, p = 0.00574). After adjusting for all covariates in Model II, the OR value does not change much compared to Model I(OR 1.380, 95% CI 1.085–1.754, p = 0.00857). We stratified the analysis by gender and BMI (Table 4). The results showed that among those infected with hepatitis B, males and those with a high BMI had a higher risk of developing periodontitis.
Discussion
We conducted a cross-sectional study of the U.S. population using data from the NHANES database. We included a total of 5,957 participants and showed a positive association between hepatitis B infection and periodontitis. This positive association persisted after adjusting for covariates (sex, age, race, education, PIR, smoking, alcohol consumption, BMI, AST, ALT, creatinine, hypertension, and diabetes). The adjusted odds ratio of hepatitis B to periodontitis suggests that hepatitis B infection is a risk factor for the development of periodontitis.
In some previous studies, the potential mechanism by which hepatitis B infection affects periodontitis was discussed. Studies conducted between 1984 and 1985 focused on detecting HBV antigens (or viral particles) in the oral fluid of infected patients. Periodontal tissue is connected to the rest of the body by blood and lymph [7]. Viral particles circulate in the blood, reach the lymphatic system, and end up in the GCF due to differences in osmotic pressure. GCF is then used as a carrier to reach the saliva [34]. This hypothesis is further supported by a recent study by Kamimura et al., who concluded that the detection of hepatitis B virus DNA is positively correlated with the detection of hepatitis B virus in salivary occult blood [35]. In addition, Amir et al. tested the levels of ALT and AST in women with chronic periodontitis and found a correlation between periodontal index and serum liver enzyme levels [36]. Concurrently, AST and ALT serve as sensitive markers for liver damage and are also indicative of the progression of viral hepatitis [37].
From an immunological perspective, enzyme-linked immunosorbent assay (ELISA) performed on saliva samples from HBV-infected patients showed significantly increased levels of pro-inflammatory interleukin (IL-2 and IL-4) and anti-inflammatory interleukin (IL-10) in saliva samples from HBV-infected patients compared to healthy controls [38]. Gharavi et al. also showed that the same immunoassay (ELISA) has good sensitivity and specificity for the diagnosis of HBV infection in saliva samples [39]. Thus, an imbalance in inflammatory mediators may support a bidirectional relationship between hepatitis B infection and periodontitis.
The conclusion of the retrospective study by Nagao et al. also highlights that periodontitis may be associated with the progression of viral liver disease; Therefore, the control of oral diseases is essential for the prevention and management of liver fibrosis [20]. They found obesity as a risk factor for periodontal disease among patients with viral liver disease [20]. This is consistent with the results of our stratified analysis. With the increase in BMI, the risk of periodontitis in hepatitis B patients increased significantly. The meta-analysis showed a significant association between obesity and periodontitis (OR = 1.30 [95% Confidence Interval (CI), 1.25–1.35]) and with mean Body Mass Index (BMI) and periodontal disease (mean difference = 2.75) [40]. Obesity is also a key component of metabolic syndrome and is associated with an increased relative risk of cancer in many tissues, including the liver [41]. Concurrently, obesity, diabetes, and metabolic syndrome have the potential to expedite the advancement of liver disease in individuals with chronic hepatitis B virus infection, ultimately leading to the onset of cirrhosis and potentially hepatocellular carcinoma [42]. In addition, periodontitis is also associated with metabolic syndrome [43].
In the clinical study of the relationship between liver disease and oral disease. The compromised periodontal health observed in individuals with hepatitis C infection may stem from liver dysfunction, weakened immune responses, or a diminished inclination among infected patients to pursue dental treatment [22, 23]. Numerous scholarly investigations have examined the impact of HCV infection on the oral cavity, emphasizing the dental pathological alterations and additional extrahepatic manifestations (EHMs) that have implications for oral health [44]. Yang et al. conducted a prospective study on the link between tooth loss and the incidence of primary liver cancer, showing that an increased number of lost teeth is associated with a higher risk of primary liver cancer [45]. The study by Dong-hun Han et al. also found that viral hepatitis may be associated with methyl mercaptan-defined halitosis [46]. We have reason to think that liver disease may harm the oral environment.
We used the NHANES database from the United States. Due to the large sample size of the data, it can well represent the adult respondents related to chronic hepatitis B infection and periodontitis in the United States. Compared with previous clinical studies, our research results are more objective and representative to a certain extent. In a previous meta-analysis, it was noted that HBV infection and its oral effects mainly involved the detection of viral antigens in saliva and gingival fluid, and were less involved in the clinical, dental, or periodontal status of infected patients [19]. Therefore, our cross-sectional study makes up for this deficiency to some extent and is an important supplement to the existing knowledge base.
However, despite these advantages, our study still has some limitations. First, because this is a cross-sectional study, cause-and-effect relationships cannot be confirmed. Second, due to the limited research on hepatitis B and periodontitis, the scope of discussion is limited. We are unable to explain the higher risk of periodontitis in men with hepatitis B in the study, and the mechanism needs to be further studied. Moreover, this is even though the 2012 AAP/CDC case definition of periodontitis has been used in the past as a global standard for epidemiological studies of periodontal disease [47]. In 2018, the European Federation of Periodontology/American Academy of Periodontology (EFP/AAP) published a new classification of periodontitis and called for it to replace the 2012 CDC/AAP Case Definition of periodontitis [48, 49]. CAL is recognized as the gold standard for periodontitis severity and progression, but it may be difficult to distinguish between incipient periodontitis and gingivitis when used alone [47]. However, the cause of CAL has not been documented in the NHANES periodontitis database. Therefore, using the 2018 EFP/AAP classification of periodontal diseases to analyze NHANES periodontitis data may lead to an increase in the prevalence of periodontitis [50, 51]. CAL and PD were used in the 2012 AAP/ CDC case definition of periodontitis to minimize the potential erroneous influence of periodontal retreat on the results of probing depth measurements [47]. In addition, due to the missing data of some variables in the NHANES database, in order to ensure the robustness of the results, we included all covariables and cleaned the data, leaving only 419 hepatitis B infection positive samples. The sample size of HBV positive is relatively small, which may affect the analysis results. Finally, because this is a large national survey, there may be confounding factors that could affect our results due to measurement errors and unmeasured variables. Therefore, this study cannot reflect the relationship between hepatitis B infection and periodontitis, so more relevant variables should be considered in the future to conduct longitudinal studies.
Conclusions
This cross-sectional study suggested that hepatitis B infection is a risk factor for periodontitis. Individuals infected with hepatitis B may be at an increased risk for periodontal diseases, necessitating enhanced oral health surveillance and care. Our study suggests that special attention should be given to men and obese individuals, who may be particularly vulnerable to severe periodontal conditions. Regular oral health examinations and proactive periodontal interventions could potentially mitigate these risks. The pathophysiological mechanism needs further study.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/.
Abbreviations
- NHANES:
-
The national health and nutrition examination survey
- HBV:
-
Hepatitis B virus
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- PIR:
-
Poverty income ratio
- BMI:
-
Body mass index
- CDC:
-
The Centers for Disease Control and Prevention
- NCHS:
-
The national center for health statistics
- AAP:
-
American Association of Periodontitis
- CI:
-
Confidence interval
- OR:
-
Odds ratio
- AL:
-
Attachment loss
- PD:
-
Pocket depth
References
Wu L, Zhang SQ, Zhao L, Ren ZH, Hu CY. Global, regional, and national burden of periodontitis from 1990 to 2019: results from the Global Burden of Disease study 2019. J Periodontol. 2022;93(10):1445–54.
Nomura Y, Okada A, Hanada N. Future prospective of oral Microbiome Research. Appl Sci. 2022;12(1):55.
Schulz S, Stein JM, Schumacher A, Kupietz D, Yekta-Michael SS, Schittenhelm F, Conrads G, Schaller H-G, Reichert S. Nonsurgical Periodontal Treatment options and their impact on Subgingival Microbiota. J Clin Med. 2022;11(5):1187.
Mombelli A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol 2000. 2018;76(1):85–96.
Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–20.
Bosshardt DD. The periodontal pocket: pathogenesis, histopathology and consequences. Periodontol 2000. 2018;76(1):43–50.
Matsumoto Y, Zhang B, Kato S. Lymphatic networks in the periodontal tissue and dental pulp as revealed by histochemical study. Microsc Res Tech. 2002;56(1):50–9.
Cecoro G, Annunziata M, Iuorio MT, Nastri L, Guida L. Periodontitis, Low-Grade inflammation and systemic health: a scoping review. Med (Kaunas) 2020, 56(6).
Beck JD, Papapanou PN, Philips KH, Offenbacher S. Periodontal Medicine: 100 years of Progress. J Dent Res. 2019;98(10):1053–62.
Bui FQ, Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, Asadi H, Ojcius DM. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42(1):27–35.
Proctor DM, Shelef KM, Gonzalez A, Davis CL, Dethlefsen L, Burns AR, Loomer PM, Armitage GC, Ryder MI, Millman ME, et al. Microbial biogeography and ecology of the mouth and implications for periodontal diseases. Periodontol 2000. 2020;82(1):26–41.
Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000 2005, 38:135–187.
Corstjens PL, Abrams WR, Malamud D. Saliva and viral infections. Periodontol 2000. 2016;70(1):93–110.
Teles F, Collman RG, Mominkhan D, Wang Y. Viruses, periodontitis, and comorbidities. Periodontol 2000. 2022;89(1):190–206.
Moutsopoulos NM, Konkel JE. Tissue-specific immunity at the oral mucosal barrier. Trends Immunol. 2018;39(4):276–87.
Tu T, Block JM, Wang S, Cohen C, Douglas MW. The lived experience of Chronic Hepatitis B: a broader view of its impacts and why we need a cure. Viruses 2020, 12(5).
Chang MH. Hepatitis B virus infection. Semin Fetal Neonatal Med. 2007;12(3):160–7.
Rizzo GEM, Cabibbo G, Craxì A. Hepatitis B Virus-Associated Hepatocellular Carcinoma. Viruses. 2022;14(5):986.
Gheorghe DN, Bennardo F, Popescu DM, Nicolae FM, Ionele CM, Boldeanu MV, Camen A, Rogoveanu I, Surlin P. Oral and Periodontal implications of Hepatitis Type B and D. Current State of Knowledge and Future perspectives. J Pers Med 2022, 12(10).
Nagao Y, Kawahigashi Y, Sata M. Association of Periodontal Diseases and Liver Fibrosis in patients with HCV and/or HBV infection. Hepat Mon. 2014;14(12):e23264.
Tomofuji T, Ekuni D, Irie K, Azuma T, Tamaki N, Maruyama T, Yamamoto T, Watanabe T, Morita M. Relationships between periodontal inflammation, lipid peroxide and oxidative damage of multiple organs in rats. Biomed Res. 2011;32(5):343–9.
Coates EA, Brennan D, Logan RM, Goss AN, Scopacasa B, Spencer AJ, Gorkic E. Hepatitis C infection and associated oral health problems. Aust Dent J. 2000;45(2):108–14.
Carrozzo M. Oral health in patients with hepatitis C virus infection: an underestimated problem? Oral Dis. 2001;7(5):267–70.
Tomofuji T, Ekuni D, Yamanaka R, Kusano H, Azuma T, Sanbe T, Tamaki N, Yamamoto T, Watanabe T, Miyauchi M, Takata T. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. J Periodontol. 2007;78(10):1999–2006.
Furusho H, Miyauchi M, Hyogo H, Inubushi T, Ao M, Ouhara K, Hisatune J, Kurihara H, Sugai M, Hayes CN, et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. J Gastroenterol. 2013;48(11):1259–70.
NCHS Ethics Review Board (ERB). Approval* [https://www.cdc.gov/nchs/nhanes/irba98.htm].
Badawy R, El-Mowafy O, Tam LE. Fracture toughness of chairside CAD/CAM materials - alternative loading approach for compact tension test. Dent Mater. 2016;32(7):847–52.
Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–54.
Burns GS, Thompson AJ. Viral hepatitis B: clinical and epidemiological characteristics. Cold Spring Harb Perspect Med. 2014;4(12):a024935.
Al-Mekhaizeem KA, Miriello M, Sherker AH. The frequency and significance of isolated hepatitis B core antibody and the suggested management of patients. CMAJ. 2001;165(8):1063–4.
Liang X, Fan J. The utilization of accurate body mass index classification is imperative for grouping based on BMI. Hum Reprod. 2022;37(3):622–3.
Glossary Adult tobacco use information [https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm].
National Health and Nutrition Examination Survey-Alcohol Use. [https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/ALQ_F.htm].
Lamster IB, Ahlo JK. Analysis of Gingival Crevicular Fluid as Applied to the Diagnosis of Oral and Systemic Diseases.
Kamimura H, Watanabe J, Sugano T, Kohisa J, Abe H, Kamimura K, Tsuchiya A, Takamura M, Okoshi S, Tanabe Y, et al. Relationship between detection of hepatitis B virus in saliva and periodontal disease in hepatitis B virus carriers in Japan. J Infect Chemother. 2021;27(3):492–6.
Ahmadinia AR, Pakkhesal M, Vakili MA. Evaluation of serum alanine aminotransferase and aspartate aminotransferase enzyme levels in women patients with chronic periodontitis. Health Care Women Int. 2022;43(1–3):367–75.
Vasconcelos DF, Pereira da Silva FR, Pinto ME, Santana LA, Souza IG, Miranda de Souza LK, Oliveira NC, Ventura CA, Novaes PD, Barbosa AL, et al. Decrease of Pericytes is Associated with Liver Disease caused by ligature-Induced Periodontitis in rats. J Periodontol. 2017;88(2):e49–57.
Azatyan V, Yessayan L, Shmavonyan M, Melik-Andreasyan G, Perikhanyan A, Porkshenyan K. Evaluation of IL-2, IL-10, IL-4 and ɤ-interferon levels in the oral fluids of patients with hepatitis C, B and HIV. J Infect Dev Ctries. 2019;13(51):s69–74.
Gharavi M, Esmaeili M. Evaluation of HBs-Ag and anti-HBc levels in serum and saliva of patients with hepatitis B. Med J Islamic Repub Iran 2020.
Moura-Grec PG, Marsicano JA, Carvalho CA, Sales-Peres SH. Obesity and periodontitis: systematic review and meta-analysis. Cien Saude Colet. 2014;19(6):1763–72.
El-Serag HB, Kanwal F. Obesity and hepatocellular carcinoma: hype and reality. Hepatology. 2014;60(3):779–81.
Wang CC, Tseng TC, Kao JH. Hepatitis B virus infection and metabolic syndrome: fact or fiction? J Gastroenterol Hepatol. 2015;30(1):14–20.
Watanabe K, Cho YD. Periodontal disease and metabolic syndrome: a qualitative critical review of their association. Arch Oral Biol. 2014;59(8):855–70.
Alavian SM, Mahboobi N, Mahboobi N, Karayiannis P. Oral conditions associated with hepatitis C virus infection. Saudi J Gastroenterol. 2013;19(6):245–51.
Yang B, Petrick JL, Abnet CC, Graubard BI, Murphy G, Weinstein SJ, Männistö S, Albanes D, McGlynn KA. Tooth loss and liver cancer incidence in a Finnish cohort. 2017, 28(8):899–904.
Han D-H, Lee S-M, Lee J-G, Kim Y-J, Kim J-B. Association between viral hepatitis B infection and halitosis. Acta Odontol Scand. 2014;72(4):274–82.
Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000. 2020;82(1):257–67.
Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159–72.
Botelho J, Machado V, Proença L, Mendes JJ. The 2018 periodontitis case definition improves accuracy performance of full-mouth partial diagnostic protocols. Sci Rep. 2020;10(1):7093.
Germen M, Baser U, Lacin CC, Fıratlı E, İşsever H, Yalcin F. Periodontitis Prevalence, Severity, and risk factors: a comparison of the AAP/CDC Case Definition and the EFP/AAP classification. Int J Environ Res Public Health 2021, 18(7).
Costea CA, Christodorescu R, Soancă A, Roman A, Micu IC, Stratul ȘI, Rusu D, Popescu DM, Popa-Wagner A, Bulboacă AE. Periodontitis in ischemic stroke patients: Case Definition challenges of the new classification Scheme (2018). J Clin Med 2022, 11(3).
Acknowledgements
We would like to thank the NHANES databases for making this data available.
Funding
This study was supported by the Science and Technology Plan Project of Guiyang in 2014 ([2014]033) and the Science and Technology Plan Project of Guiyang in 2024 ([2024]2–30).
Author information
Authors and Affiliations
Contributions
XR.C. designed the research study, analyzed the data, and wrote the manuscript. Y.L., Z.C., JK.S.,JL.S., and SY.L. supervised the study and revised the manuscript. JQ.Z., XJ.C., CW.Z., JX.H., XT.C., and FQ.J. analyzed the data and drafted the results sections. All authors participated in the interpretation and discussion of the results and gave final approval of the manuscript to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All study participants gave written informed consent to the National Center for Health Statistics Research Ethics Review Board (Protocol #2009–14) and study ethics guidelines at the Centers for Disease Control and Prevention. Parental consent was obtained for participants aged 15 and below.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, X., Song, J., Sun, J. et al. Hepatitis B infection is associated with periodontitis: the national health and nutrition examination survey (2009–2014). BMC Oral Health 24, 815 (2024). https://doi.org/10.1186/s12903-024-04489-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04489-y