Abstract
Background
Studies investigating thermocycling effect on surface topography and fracture toughness of resins used in digitally manufactured denture bases are few. The study aimed to assess the impact of thermocycling on surface topography and fracture toughness of materials used for digitally manufactured denture bases.
Methods
Water sorption, solubility, hardness, surface roughness, and fracture toughness of both three-dimensional (3D)-printed and computer-aided design, computer-aided manufacturing (CAD-CAM) milled specimens (n = 50) were assessed both prior to and following 2000 thermocycles, simulating 2 years of clinical aging. Surface hardness (n = 10) was measured using a Vickers hardness testing machine, surface roughness (n = 10) was determined by a contact profilometer, and fracture toughness (n = 20) was measured using the 3-point bend test, then studying the fractured surfaces was done via a scanning electron microscope (SEM). Prior to and following thermocycling, water sorption and solubility (n = 10) were assessed. Normally distributed data was tested using two-way repeated ANOVA and two-way ANOVA, while Mann Whitney U test and the Wilcoxon signed ranks test were used to analyze data that was not normally distributed (α < 0.05).
Results
Following thermocycling, Vickers hardness and fracture toughness of both groups declined, with a significant reduction in values of the 3D-printed resin (P < .001). The 3D-printed denture base resins had a rougher surface following thermocycling with a significant difference (P < .001). The sorption and solubility of water of both materials were not affected by thermocycling.
Conclusions
Before and after thermocycling, milled specimens had lower surface roughness and a greater degree of hardness and fracture toughness than 3D-printed specimens. Thermocycling lowered hardness and fracture toughness, and increased surface roughness in both groups, but had no effect on water sorption and solubility.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Complete dentures (CDs) have been made using the traditional compression mold or the flask-pack-press for more than 50 years. Polymethylmethacrylate (PMMA) resin and heat polymerization have been used to produce CDs [1].
The advancement of CAD-CAM processes for CDs, however, has caused a notable revolution in this protocol in recent years [2]. In contrast to conventional methods, CAD-CAM dentures may be constructed without the need for time-consuming labor-intensive processes [3,4,5,6]. Digital methods have the advantage of faster denture fabrication and fewer stages in the process, which can lower the likelihood of errors [7, 8].
The process of creating digital dentures was first established as a subtractive technique in which the dentures were manufactured from pre-polymerized resin blocks. Research has concluded that milled resins exhibit higher surface and mechanical properties [4, 9,10,11,12], reduced microbial colonization [13, 14], a decreased leach rate of residual monomer [15], and comparable color stability [16,17,18], in comparison to compression molded resins, because controlled conditions and high pressure were used during manufacturing of pre-polymerized blocks.
Later, the additive approach was introduced, in which digital technology was used to manufacture dentures layer by layer utilizing 3D printing technology and liquid resins polymerized using ultraviolet light [19]. Dentistry has made use of a variety of 3D printers and materials, such as fused deposition modeling (FDM), thermal inkjet (TIJ), and selective laser sintering (SLS) [20]. This method is more cost-effective because it allows for the simultaneous production of several products and eliminates the need for rotary tool wear and raw material waste [21, 22].
It is crucial to evaluate the surface qualities and mechanical characteristics of CAD-CAM materials to guarantee their success [23,24,25,26,27]. The wettability, hardness, and surface properties of acrylic resins may have an impact on plaque buildup [28, 29]. Dentures are constantly exposed to temperature fluctuations brought on by food and beverages [30, 31]. The characteristics of dental materials may be negatively impacted by these temperature fluctuations, especially if they are experienced frequently [30]. As a result, it is critical to evaluate denture base material performance in settings that mimic the intraoral environment [32].
Due to temperature variations brought on by ingested foods and beverages, the intraoral environment is a thermally dynamic medium [24]. The oral cavity’s temperature fluctuates 20 to 50 times a day, or 10,000 times a year on average [32] A recognized technique for subjecting dental materials to water baths with varying temperatures and simulating intraoral temperature changes is thermal cycling at 5° to 55 °C for 30 s [30, 33]. The surfaces of dentures may degrade as a result of thermal stresses brought on by these temperature variations [32, 34].
Research has shown that CAD-CAM milled resins have improved properties compared with 3D-printed resins such as higher fracture toughness, lower water sorption [35,36,37], and higher flexural modulus [38], while another study showed higher fracture toughness for 3D-printed resins [39]. Additional research [40,41,42,43] focused on the color stability and roughness of the surface of denture base resins. There is insufficient data on how thermal stresses affect the surface characteristics and fracture toughness of digitally manufactured resins used for denture bases, thus it is important to test the effect of thermal stresses to have a better prediction of which type will have a greater resistance to thermal changes and is better suited for long term prostheses clinically.
The aim of our study is to evaluate the effect of thermal cycling on Vickers hardness, surface roughness, fracture toughness, sorption of water, and solubility of denture base materials that have been CAD-CAM milled and 3D-printed. The null hypothesis was that thermocycling would have no effect on the tested properties of 3D-printed and CAD-CAM milled denture base materials.
Methods
The Committee of Research Ethics in Alexandria University, Faculty of Dentistry (IORG 0008839) has approved the research prior to any research-related activities.
Two groups (n = 50) were investigated in this study, 3D-printed resin (Denture base LP; Formlabs) and prepolymerized blanks (M-PM; Merz Dental GmbH), (Table 1). The estimated sample size was 100 specimens, based upon the assumptions of a 95% level of confidence and an 80% study power [32, 44]. A computer program (G*power 3.0.10; Heinrich Heine University Düsseldorf) [45] and Rosner technique [46] was used to calculate the sample size.
For each material, Vickers hardness (n = 10), surface roughness (n = 10), fracture toughness(n = 20), solubility, and sorption (n = 10) were evaluated before and after thermocycling. The specimens were created using a software program (Autodesk Meshmixer; Autodesk Inc.) and stored as standard tessellation language (STL) files.
Specimens’ dimensions
Specimens for Vickers hardness with measurements of 15 × 10 × 2.5 mm were fabricated in accordance with Organization for International Standardization (ISO) 20795-1:2013 [47]. For the surface roughness test, the specimens were disk shaped with dimensions of 10 × 2 mm [18]. In order to test for fracture toughness, specimens with measurements of 39 × 8 × 4 mm dimensions and a 3.2 mm precrack were manufactured in accordance with ISO 20795-1 [47]. Circular specimens with 15 × 2 mm size were fabricated to test the solubility and sorption of materials in water [48].
Specimens’ fabrication
In the CAD-CAM milled group, the specimens were milled to the specified dimensions (Fig. 1) from PMMA-based blanks using a computer numeric controlled (CNC) milling machine (Ceramill motion 2; AmannGirrbach). The specimens made by 3D printing (Fig. 2) were constructed by sending the files to a 3D printing machine (Form 3; Formlabs). Biocompatible denture base resin (Denture base OP; Formlabs) was used for printing at an angle of 45-degrees to the printing platform with 50 μm thickness of each layer [10], and the software program used for the printer (PreForm; Formlabs) was used to add supporting structures. Cleaning of the specimens was done using 99% isopropyl alcohol for 5 min in an ultrasonic bath (Sonorex RK; Bandelin) to remove any uncured or excess resin according to instructions stated by the manufacturer. The specimens were then submerged in glycerin and polymerized at 80 °C for 30 min using ultraviolet light in a postpolymerization lightbox (Form Cure; Formlabs) [35].
Silicon disks (CarbiMet; Buehler Ltd) were used to finish the specimens. A slurry of pumice (Pumice medium; Industrial plaster Ltd) with a lathe brush and a polishing paste (Universal polishing paste; Renfert GmbH) applied at a speed of 500 rpm [49] for 90 s, were used to polish the specimens. A single experienced operator completed the finishing and polishing procedures. The polishing of specimens was done according to manufacturer’s instructions, and this is recommended clinically to achieve a smooth denture surface.
Thermal cycling
In accordance with ISO 20795-1 [47], the samples were kept in distilled water at 37 °C for 50 h (baseline). Half of the specimens then underwent 2000 cycles of thermal aging in a thermocycler (THE-1100; SD Mechatronik) to simulate the clinical aging of denture base materials for 2 years, with immersion times of 30 s in water that was 5 °C/55°C and dwell times of 10 s [50].
Vickers hardness test
All the tested properties were assessed preceding and following thermocycling. Vickers hardness testing machine (FM-700, Future Tech Corp) was used to measure the hardness of the specimens (n = 10 per group). A diamond-shaped indenter was used to apply 50 g with a dwell period of 30 s for each specimen, 3 indentations were made, and the average was obtained [51]. The diagonals of the diamond-shaped indentations (Fig. 3) were measured to the closest 0.1 μm. The Vickers hardness number (VHN) was calculated using the formula: VHN = 0.185 L/d2, where L stands for load (N) and d for mean length of the diagonal (µm) [28].
Surface roughness measurement
The contact profilometer (Surftest SV-3100, Mitutoyo Corp) was utilized to measure the specimens’ surface roughness (Ra) with a stylus speed of 1 mm/second and an endpoint of 0.8 mm (n = 10 per group). Three readings were obtained for each specimen, and the average was determined in micrometers (µm) [10, 50].
Fracture toughness test
The fracture toughness test was conducted (n = 20 for each group) using the 3-point bend method using an all-purpose testing machine (Omnitest; Macmesin) with a 32 mm wide span and a rate of displacement of 1 mm/minute before thermocycling [47]. The test was conducted on 10 samples before thermocycling, and 10 other samples after thermocycling, because the specimens fracture during the test. Using the following equations [52], the fracture toughness (KIc) was calculated from the recorded highest force:
\(f\left(x\right)=\frac{{3x}^{\frac{1}{2}} [1.99-x\left(1-x\right)(2.15-3.93x+2.7{x}^{2}]}{\left[2\left(1+2x\right){\left(1-x\right)}^{\frac{3}{2}} \right]}, x=\frac{a}{{h}_{t}}\),
where Fmax is the specimen maximum applied force expressed in Newtons, a represents the precrack length expressed in mm, ht denotes the height of specimen expressed in mm, bt denotes the width of the specimen mm, and lt represents the width of the span in mm. To assess the cracked surface utilizing a SEM (JSM 200 IT, JOEL) at magnification levels (×100) and (×300), specimens from the 2 groups were sputtered with gold.
Water sorption and solubility test
Circular specimens (n = 10 per group) were dehydrated using silica gel at 37 °C until a consistent mass was observed for water solubility and sorption. The specimen weights were determined utilizing an analytical scale (AS 220.R2, RADWAG) with a precision of 0.1-mg precision. By employing a digital micrometer (Digimatic, Mitutoyo), the dimensions of each specimen were computed by taking the average of the 3 measurements taken on each side while the specimens’ mass remained fixed (m1) [53].
Specimens were weighed again (m2) following 7 days of submersion inside water at a temperature of 37 °C (baseline). Following 2000 thermocycles to assess the sorption with time, specimens were weighed (m3). Once an unaltered mass (m4) was attained, the specimens were once more dried in the desiccator. In line with ISO 4049 [54], calculating solubility (Wsl) and sorption (Wsp) involved use of the following formula:
Wsp 7 days = \(\frac{m2-m1}{V}\) Wsp thermocycling = \(\frac{m3-m1}{V}\) Wsl = \(\frac{m4-m1}{V}\), where m is the dry specimen mass in mg before being submerged in water, m2 is the specimen mass following 7 days immersed in water and is measured in mg, m3 is the specimen mass following thermocycling in mg, m4 is the specimen mass following the 2nd drying in mg, and V is the specimen volume in mm3.
Q-Q plots and the Shapiro-Wilk test were used to verify the normality of the variables. Since the values for surface roughness, Vickers hardness, and fracture toughness were all normally distributed, the means, standard deviations, and 95% confidence intervals (CI) were used to present the data. However, sorption of water and solubility showed non normal distribution hence it was presented mainly using median, interquartile range (IQR), and minimum and maximum values. Percent change in all parameters was calculated based on the formula: [(values after thermocycling – values before thermocycling) / values before thermocycling] x 100. To clarify how denture material and thermocycling, as well as their combined effects, affect fracture toughness, a two-way ANOVA was used, while 2 -way repeated measures ANOVA were performed to analyze Vickers hardness and surface roughness. The Wilcoxon Sign Rank test was used to evaluate variations in water sorption within groups, and the Mann Whitney U test was used for comparing water sorption, solubility, and percent change. P value < 0.05 was used as the significance level, and all tests were two-tailed. IBM SPSS version 23, Armonk, NY, USA, was used to analyze the data.
Results
Results revealed that Vickers hardness of CAD-CAM milled denture base material was higher compared with 3D-printed resins before (18.02 ± 0.67 and 16.26 ± 0.79, respectively) and after thermal cycling (16.26 ± 0.79 and 12.42 ± 1.30, respectively). Both groups showed a decline in Vickers hardness following thermocycling (Fig. 4). The two-way repeated ANOVA displayed a significant effect of the denture base material (P < .001), thermocycling (P < .001), and a significant interaction of both factors affecting Vickers hardness (P = .001).
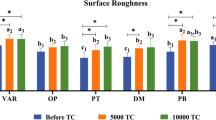
The values of roughness of the surface of the 3D-printed resin were greater compared to CAD-CAM milled group both prior to (0.69 ± 0.05 and 0.18 ± 0.01, respectively) and following thermocycling (1.16 ± 0.16 and 0.14 ± 0.02, respectively). The surface roughness of both groups significantly increased following thermocycling (Fig. 5). There was significant effect of denture base material (P < .001), thermocycling (P < .001), as well as their significant interaction (P < .001) on surface roughness as revealed by repeated two-way repeated ANOVA test.
Fracture toughness values of CAD-CAM milled group were higher compared with 3D-printed group before (4.16 ± 0.06 and 1.30 ± 0.06, respectively) and after thermocycling (3.82 ± 0.08 and 0.78 ± 0.05, respectively). Thermocycling significantly decreased the fracture toughness of both groups (Fig. 6). There was significant effect of denture base material (P < .001), thermocycling (P < .001), and a significant effect of their interaction (P < .001) on fracture toughness as revealed by the two-way ANOVA test.
SEM images were taken for the internal fractured surfaces of specimens initiated by the pre-crack after the three-point bending test and revealed a compact structure in the CAD-CAM milled group with small irregular cracks and a jagged structure indicating slow crack propagation (Fig. 7). However, images of the 3D-printed group after thermocycling revealed a layered structure with sharp and wide cracks indicating fast crack propagation and delamination of layers (Fig. 8).
No difference was found in water sorption of CAD-CAM milled denture base prior to and following thermocycling (P = .154). However, water sorption of 3D-printed denture base resins decreased after thermocycling with a significant difference (P = .020). Water sorption of the 3D-printed group was greater before thermocycling with a significant difference (P = .003) However, after thermocycling the water sorption of both groups was similar (P = .562). Furthermore, the water solubility of both materials showed no significant difference (P = .759) (Table 2).
Table 3 shows the percent changes in values of Vickers hardness, surface roughness, fracture toughness, and water sorption and solubility after thermocycling in both groups. The percent change of hardness in the 3D-printed resin (-23.09 (14.72)) was higher than the milled resin (-11.29 (5.15)) with a statistically significant difference (P = .001). The percent changes of surface roughness and fracture toughness in the 3D-printed group were significantly greater than the milled group (P < .001). The percentage change in water sorption after thermocycling showed no significant difference between the 2 groups (P = .644).
Discussion
The goal of the present study was to test how thermocycling affected the surface topography and fracture toughness of denture base materials constructed by 3D printing and milling via CAD-CAM. The null hypothesis was partially rejected as results revealed that thermocycling influenced surface topography and fracture toughness and there was a difference between the two materials but there was no difference regarding water sorption and solubility.
Artificial thermocycling is used to mimic temperature changes in the oral cavity, imitating prolonged usage of dentures in the oral environment under temperature variations [35].
Hardness refers to the ability of a material to withstand localized plastic deformation caused by abrasion or mechanical indentation. Dentures with low surface hardness are susceptible to damage from mechanical brushing, which can lead to pigmentations and plaque retention, shortening their lifespan [3].
In the present study, Vickers hardness of both milled and 3D-printed resin decreased following thermal cycling, however, the milled group values continued to be higher than the 3D-printed group. Furthermore, in comparison to the milled group, the 3D-printed group’s percentage change in Vickers hardness was noticeably higher. These results are consistent with Gad et al. [24], who found that 3D-printed resins used for denture bases had lower hardness values after thermocycling. This could be due to water uptake, which is a phenomenon that depends on temperature. The water, which is absorbed serves as a plasticizing agent, causing inter-chain bond cleavage and subsequently deterioration of mechanical properties which is further increased due to heat that may cause delamination of 3D printed layers [49]. This can be attributed to the microstructure of 3D printed denture base resins due to the layered structure as seen in SEM images. This is not seen in milled denture base resins because of the compact microstructure due to pre-polymerization under heat and pressure.
The ability of resin materials to absorb water is a process that is controlled by diffusion that happens either when it enters spaces like micro-voids or when it interacts with certain molecules [40]. The interaction is dependent on resin polarity, or the quantity of polar sites open to hydrogen bonding with water [27]. Residual monomer may elute, and internal stresses are released with time causing cracks to form with time which may influence the characteristics of the denture base resin [26]. Since they reflect the material resistance to the surrounding oral fluids, water sorption and solubility are important when evaluating the denture base durability [53].
In the present study, thermocycling did not affect water sorption of the CAD-CAM milled group, while the 3D-printed group displayed a different behavior and there was a significant difference after thermocycling. These results are similar to Berli et al. [53], who found that thermocycling had a significant effect on water sorption of 3D-printed materials but did not affect pressed and milled resins. However, our results are opposite to Gad et al. [25], who found that thermal cycling resulted in a significant increase of water sorption of the studied materials. This may be due to the different tested materials and decreased amount of residual monomer due to different post-printing polymerization parameters.
When comparing both groups, the water sorption of the 3D-printed group was significantly greater in comparison with the milled group before thermocycling. These findings are similar to Perea-Lowery et al. [48], Berli et al. [53], Hanno et al. [35], and Dimitrova et al. [36], who also found that 3D-printed resins exhibit higher water sorption, due to increased residual monomers.
Changes in intraoral temperature when fluids are present may cause acrylic resin to absorb water more quickly and weaken the qualities of the polymer [55]. Moreover, water molecules fill the spaces between polymer chains as they diffuse into the polymer mass, pushing them apart and influencing the mechanical properties in the process [55]. Furthermore, this phenomenon is made worse by the thermal stresses that higher temperatures represent, which further deteriorate the properties of polymers [56].
Surface roughness of denture base resins is very important because denture bases are in contact with oral tissues. Surface roughness values of both groups in the study increased after thermocycling. Denture base resin that was 3D-printed had rougher surfaces than resin that was CAD-CAM milled following thermocycling with a significant difference. The surface roughness values of milled resin did not exceed the clinically acceptable threshold of 0.2 μm [29]. These results are similar to that of Çakmak et al. [28], who found that roughness of CAD-CAM resins was lower than that of 3D-printed denture base materials after thermocycling. CAD-CAM dentures are fabricated from prepolymerized PMMA resin under heat and pressure which leads to less residual monomer and higher degree of polymerization with less voids and spaces. 3D-printed dentures, on the other hand, are printed one layer at a time and then polymerization follows printing. This may lead to a weak inter-layer bonding due to unreacted remaining monomers and thus a minimal level of polymerization with more voids and inter-layer spacings creating a rough surface [10]. Specimens were printed at an angle of 45º and this caused an increase in surface roughness values due to height of step edges and stepwise connection between layers [57].
The degree to which a material is resistant to the propagation of defects under an applied load is measured using its fracture toughness [58]. It represents the maximum amount of stress that a material can withstand before a crack starts and spreads throughout it [59]. One method that is frequently used is three-point bending of a sharply notched beam to ascertain the fracture toughness of denture bases [60]. A denture base material can withstand cracking if it has a high fracture toughness [58].
In the present study, fracture toughness of both groups decreased after thermocycling, however, the percentage decrease in the 3D-printed group was 5 times greater than the milled group with a significant difference. These findings contrast with Mann et al. [39], who found that 3D-printed denture base resin had greater fracture toughness than milled resin. This may be because they tested specimens without thermocycling. SEM images confirmed fracture results with images showing wide and long fractures in 3D-printed resin, which is due to delamination of layers, facilitated by heat.
Limitations of this study include the use of milled and 3D-printed materials from single manufacturers, and the use of relatively low number of thermocycles. Future investigation is needed to study materials from various manufacturers and to test denture base materials under higher number of thermocycles and in different aging solutions.
Conclusions
The following conclusions were reached based on the results of this in vitro study:
-
1.
Vickers hardness and fracture toughness of CAD-CAM milled and 3D-printed denture base materials decreased after thermocycling but those of CAD-CAM milled were higher with a significant difference.
-
2.
The roughness of the surfaces of both CAD-CAM milled and 3D-printed denture bases increased after thermocycling but those of CAD-CAM milled were lower with a significant difference.
-
3.
Thermocycling did not have any effect on sorption of water and solubility of both denture base materials.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 3D:
-
Three-Dimensional
- CAD-CAM:
-
Computer-aided Design, Computer-aided Manufacturing
- SEM:
-
Scanning Electron Microscope
- CDs:
-
Complete Dentures
- PMMA:
-
Polymethylmethacrylate
- STL:
-
Standard Tessellation Language
- ISO:
-
Organization for International Standardization
- CNC:
-
Computer Numeric Controlled
- VHN:
-
Vickers Hardness Number
References
Matsuo H, Suenaga H, Takahashi M, Suzuki O, Sasaki K, Takahashi N. Deterioration of polymethyl methacrylate dentures in the oral cavity. Dent Mater. 2015;34:234–9.
Srinivasan M, Kalberer N, Kamnoedboon P, Mekki M, Durual S, Özcan M, et al. CAD-CAM complete denture resins: an evaluation of biocompatibility, mechanical properties, and surface characteristics. J Dent. 2021;114:103785.
Prpić V, Schauperl Z, Ćatić A, Dulčić N, Čimić S. Comparison of mechanical properties of 3D-printed, CAD/CAM, and conventional denture base materials. J Prosthodont. 2020;29:524–8.
Al-Dwairi ZN, Tahboub KY, Baba NZ, Goodacre CJ, Özcan M. A comparison of the surface properties of CAD/CAM and conventional polymethylmethacrylate (PMMA). J Prosthodont. 2019;28:452–7.
Chhabra M, Kumar MN, RaghavendraSwamy KN, Thippeswamy HM. Flexural strength and impact strength of heat-cured acrylic and 3D printed denture base resins-A comparative in vitro study. J Oral Biol Craniofac Res. 2022;12:1–3.
Srinivasan M, Schimmel M, Naharro M, O’Neill C, McKenna G, Müller F. CAD/CAM milled removable complete dentures: time and cost estimation study. J Dent. 2019;80:75–9.
Infante L, Yilmaz B, McGlumphy E, et al. Fabricating complete dentures with CAD/CAM technology. J Prosthet Dent. 2014;111:351–5.
Bilgin M, Baytaroglu E, Erdem A, et al. A review of computer-aided design/computer-aided manufacture techniques for removable denture fabrication. Eur J Dent. 2016;10:286–91.
Srinivasan M, Gjengedal H, Cattani-Lorente M, Moussa M, Durual S, Schimmel M, et al. CAD/CAM milled complete removable dental prostheses: an in vitro evaluation of biocompatibility, mechanical properties, and surface roughness. Dent Mater. 2018;37:526–33.
El Samahy MM, Abdelhamid AM, El Shabrawy SM, Hanno KI. Evaluation of physicomechanical properties of milled versus 3D-printed denture base resins: a comparative in vitro study. J Prosthet Dent. 2023;129:797–e1.
Aguirre BC, Chen JH, Kontogiorgos ED, Murchison DF, Nagy WW. Flexural strength of denture base acrylic resins processed by conventional and CAD-CAM methods. J Prosthet Dent. 2020;123:641–6.
Al-Dwairi ZN, Tahboub KY, Baba NZ, Goodacre CJ. A comparison of the flexural and impact strengths and flexural modulus of CAD/CAM and conventional heat‐cured polymethyl methacrylate (PMMA). J Prosthodont. 2020;29:341–9.
Murat S, Alp G, Alatalı C, Uzun M. In vitro evaluation of adhesion of Candida albicans on CAD/CAM PMMA-based polymers. J Prosthodont. 2019;28:e873–9.
Al-Fouzan AF, Al-Mejrad LA, Albarrag AM. Adherence of Candida to complete denture surfaces in vitro: a comparison of conventional and CAD/CAM complete dentures. J Adv Prosthodont. 2017;9:402–8.
Ayman AD. The residual monomer content and mechanical properties of CAD\CAM resins used in the fabrication of complete dentures as compared with heat cured resins. Electron Physician. 2017;9:4766.
Al-Qarni FD, Goodacre CJ, Kattadiyil MT, Baba NZ, Paravina RD. Stainability of acrylic resin materials used in CAD-CAM and conventional complete dentures. J Prosthet Dent. 2020;123:880–7.
Gruber S, Kamnoedboon P, Özcan M, Srinivasan M. CAD/CAM complete denture resins: an in vitro evaluation of color stability. J Prosthodont. 2021;30:430–9.
Alp G, Johnston WM, Yilmaz B. Optical properties and surface roughness of prepolymerized poly (methyl methacrylate) denture base materials. J Prosthet Dent. 2019;121:347–52.
Revilla-León M, Özcan M. Additive manufacturing technologies used for processing polymers: current status and potential application in prosthetic dentistry. J Prosthodont. 2019;28:146–58.
Ventola CL. Medical applications for 3D printing: current and projected uses. Pharmacol Ther. 2014;39:704.
Kattadiyil MT, AlHelal A. An update on computer-engineered complete dentures: a systematic review on clinical outcomes. J Prosthet Dent. 2017;117:478–85.
Shim JS, Kim JE, Jeong SH, Choi YJ, Ryu JJ. Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D-printed resins with various printing orientations. J Prosthet Dent. 2020;124:468–75.
Niem T, Youssef N, Wostmann B. Energy dissipation capacities of CAD-CAM restorative materials: a comparative evaluation of resilience and toughness. J Prosthet Dent. 2019;121:101–9.
Gad MM, Fouda SM, Abualsaud R, Alshahrani FA, Al-Thobity AM, Khan SQ, et al. Strength and surface properties of a 3D‐printed denture base polymer. J Prosthodont. 2022;31:412–8.
Gad MM, Alshehri SZ, Alhamid SA, Albarrak A, Khan SQ, Alshahrani FA, et al. Water sorption, solubility, and translucency of 3D-printed denture base resins. Dent J. 2022;10:42.
Iwaki M, Kanazawa M, Arakida T, Minakuchi S. Mechanical properties of a polymethyl methacrylate block for CAD/CAM dentures. J Oral Sci. 2020;62:420–22.
Tuna SH, Keyf F, Gumus HO, Uzun C. The evaluation of water sorption/ solubility on various acrylic resins. Eur J Dent. 2008;2:191–97.
Çakmak G, Molinero-Mourelle P, De Paula MS, Akay C, Cuellar AR, Donmez MB, et al. Surface roughness and color stability of 3D-printed denture base materials after simulated brushing and thermocycling. Mater. 2022;15:6441.
Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 1997;13:258–69.
Silva CD, Machado AL, Chaves CD, Pavarina AC, Vergani CE. Effect of thermal cycling on denture base and autopolymerizing reline resins. J Appl Oral Sci. 2013;2:219–24.
Morresi AL, D’Amario M, Capogreco M, Gatto R, Marzo G, D’Arcangelo C, et al. Thermal cycling for restorative materials: does a standardized protocol exist in laboratory testing? A literature review. J Mech Behav Biomed Mater. 2014;29:295–308.
Ayaz EA, Bağış B, Turgut S. Effects of thermal cycling on surface roughness, hardness and flexural strength of polymethylmethacrylate and polyamide denture base resins. J Appl Biomater Funct Mater. 2015;13:280–6.
Tasin S, Ismatullaev A. Comparative evaluation of effect of thermocycling on the mechanical properties of conventionally polymerized, CAD-CAM milled and 3D-printed materials. J Prosthet Dent. 2022;127:173–9.
Arslan M, Murat SE, Alp G, Zaimoglu A. Evaluation of flexural strength and surface properties of prepolymerized CAD/CAM PMMA-based polymers used for digital 3D complete dentures. Int J Comput Dent. 2018;21:31–40.
Hanno KI, Abdul-Monem MM. Effect of denture cleansers on the physical and mechanical properties of CAD-CAM milled and 3D-printed denture base materials: an in vitro study. J Prosthet Dent 2023; ISSN 0022–3913.
Dimitrova M, Vlahova A, Hristov I, Kazakova R, Chuchulska B, Kazakov S, et al. Evaluation of water sorption and solubility of 3D-printed, CAD/CAM milled, and PMMA denture base materials subjected to artificial aging. J Compos Sci. 2023;7:339.
Batisse C, Nicolas E. Comparison of CAD/CAM and conventional denture base resins: a systematic review. Appl Sci. 2021;11:5990.
Grzebieluch W, Kowalewski P, Grygier D, Rutkowska-Gorczyca M, Kozakiewicz M, Jurczyszyn K. Printable and machinable dental restorative composites for cad/cam application—comparison of mechanical properties, fractographic, texture and fractal dimension analysis. Mater. 2021;14:4919.
Mann RS, Ruse ND. Fracture toughness of conventional, milled and 3D printed denture bases. Dent Mater. 2022;38:1443–51.
Al-Dwairi Z, Al Haj Ebrahim AA, Baba NZ. A comparison of the surface and mechanical properties of 3D printable denture-base resin material and conventional polymethylmethacrylate (PMMA). J Prosthodont. 2023;32:40–8.
Alfouzan AF, Alotiabi HM, Labban N, Al-Otaibi HN, Al Taweel SM, AlShehri HA. Effect of aging and mechanical brushing on surface roughness of 3D-printed denture resins: a profilometer and scanning electron microscopy analysis. Technol Health Care. 2022;30:161–73.
Di Fiore A, Meneghello R, Brun P, Rosso S, Gattazzo A, Stellini E, et al. Comparison of the flexural and surface properties of milled, 3D-printed, and heat polymerized PMMA resins for denture bases: an in vitro study. J Prosthodont Res. 2022;66:502–8.
Alfouzan AF, Alotiabi HM, Labban N, Al-Otaibi HN, Al Taweel SM, AlShehri HA. Color stability of 3D-printed denture resins: Effect of aging, mechanical brushing and immersion in staining medium. J Adv Prosthodont. 2021;13:160–71.
Gad MM, Al- Thobity AM, Fouda SM, Näpänkangas R, Raustia A. Flexural and surface properties of PMMA denture base material modified with thymoquinone as an antifungal agent. J Prosthodont. 2020;29:243–50.
Universität, Düsseldorf. G*Power.2019. Retrieved from http://www.gpower.hhu.de/.
Rosner B. Fundamentals of Biostatistics. Hypothesis testing: two-sample inference. 7th ed. Boston: Brooks/Cole. Nelson Education; 2015. pp. 269–301.
ISO;20795–1. Dentistry – Base polymers. Part 1: Denture base polymers. International Organization for Standardization; 2nd ed. Geneva, Switzerland. 2013.
Perea-Lowery L, Gibreel M, Vallittu PK, Lassila LV. 3D-printed vs. heat-polymerizing and autopolymerizing denture base acrylic resins. J Mater. 2021;14:5781.
Fernandez P, Unkovskiy A, Benkendorff V, Klink A, Spintzyk S. Surface characteristics of milled and 3D-printed denture base materials following polishing and coating: an in-vitro study. J Mater. 2020;13:3305.
Atalay S, Çakmak G, Fonseca M, Schimmel M, Yilmaz B. Effect of thermocycling on the surface properties of CAD-CAM denture base materials after different surface treatments. J Mech Behav Biomed Mater. 2021;121:104646.
Altarazi A, Haider J, Alhotan A, Silikas N, Devlin H. Assessing the physical and mechanical properties of 3D-printed acrylic material for denture base application. Dent Mater. 2022;38:1841–54.
Aati S, Akram Z, Shrestha B, Patel J, Shih B, Shearston K, et al. Effect of post-curing light exposure time on the physico–mechanical properties and cytotoxicity of 3D-printed denture base material. Dent Mater. 2022;38:57–67.
Berli C, Thieringer FM, Sharma N, Müller JA, Dedem P, Fischer J, et al. Comparing the mechanical properties of pressed, milled, and 3D-printed resins for occlusal devices. J Prosthet Dent. 2020;124:780–6.
ISO 4049. Dentistry. Polymer-based restorative materials. Geneva: International Organization for Standardization; 2009.
Lin CT, Lee SY, Tsai TY, et al. Degradation of repaired denture base material in simulated oral fluid. J Oral Rehabil. 2000;27:190–8.
Vallittu PK, Ruyter IE. The swelling phenomenon of acrylic resin polymer teeth at the interface with denture base polymers. J Prosthet Dent. 1997;78:194–9.
Li P, Fernandez PK, Spintzyk S, Schmidt F, Beuer F, Unkovskiy A. Effect of additive manufacturing method and build angle on surface characteristics and Candida albicans adhesion to 3D printed denture base polymers. J Dent. 2022;116:103889.
Ilie N, Hilton T, Heintze S, Hickel R, Watts D, Silikas N, et al. Academy of Dental materials guidance—Resin composites: part I—Mechanical properties. Dent Mater. 2017;33:880–94.
Lassila L, Keulemans F, Säilynoja E, Vallittu PK, Garoushi S. Mechanical properties and fracture behavior of flowable fiber reinforced composite restorations. Dent Mater. 2018;34:598–606.
Elbishari H, Silikas N, Satterthwaite J. Filler size of resin-composites, percentage of voids and fracture toughness: is there a correlation? Dent Mater. 2012;31:523–7.
Acknowledgements
We would like to thank Dr. Hams Abdelrahman (Assistant Lecturer in the Department of Dental and Public Health) for her assistance with statistical analysis of the data, and formulating the tables and graph figures for statistical representation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial,
or not-for-profit sectors.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.M.A. formulated the methodology, contributed to the data curation, and writing and reviewing of the manuscript.K.I.H. performed the investigation, provided the resources, prepared the manuscript, and reviewing and editing of the manuscript. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by The Research Ethics Committee, Faculty of Dentistry, Alexandria University, Egypt (international No.: IORG0008839).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The manuscript is original research work and is not derived from any dissertations or theses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abdul-Monem, M.M., Hanno, K.I. Effect of thermocycling on surface topography and fracture toughness of milled and additively manufactured denture base materials: an in-vitro study. BMC Oral Health 24, 267 (2024). https://doi.org/10.1186/s12903-024-03991-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-03991-7