Abstract
Background
Recently, a systematic review and meta-analysis demonstrated that overexpression of p53 immunoprotein was significantly associated with progression risk of oral potentially malignant disorders (OPMD). However, the results of investigations on TP53 genetic typing in OPMD were inconsistent and inconclusive.
Methods
A systematic evaluation was conducted to identify all eligible case–control studies on the association of TP53 codon 72 polymorphism with both onset and progression of OPMD.
Results
A total of 768 OPMD patients and 1173 healthy individuals were identified from 12 eligible case–control studies on TP53 codon 72 polymorphism OPMD onset. In overall and subgroup analyses, no significantly risk of OPMD onset was observed in the cases for genetic models including allele C vs. G, homozygote CC vs. GG, heterozygote GC vs. GG, dominant GC + CC vs. GG, and recessive CC vs. GG + GC (all P-value of association test > 0.05). Further, a total of 465 OPMD patients and 775 oral squamous cell carcinoma (OSCC) ones were identified from 8 eligible case–control studies on this polymorphism in OPMD progression to OSCC. The analyses revealed that there was also no significantly risk of OPMD progression in the cases for the genetic models (all P-value of association test > 0.05).
Conclusion
Our data of a pooled-analysis indicates that TP53 codon 72 polymorphism may not act as genetic factor for the risk of OPMD onset and progression. Combined with the conclusion by a systematic review and meta-analysis, we put forward a new opinion that TP53 genetic typing cloud not influence p53 protein expression in OPMD.
Similar content being viewed by others
Introduction
Oral potentially malignant disorders (OPMD) contain a group of lesions such as oral leukoplakia (OLK), oral erythroplakia, oral lichen planus, and oral submucous fibrosis, which carry a significantly increased risk for malignant progression to oral squamous cell carcinoma (OSCC) [1]. The onset and progression of OPMD arise as a result of a multi-step carcinogenic process that correlates to the accumulation of genetic and epigenetic alterations [2]. Single nucleotide polymorphisms (SNP) in gene encoding for susceptibility factors may influence gene expression, protein function, and disease predisposition [3]. Although the etiology and progression of OPMD are quite complex, evidence indicates that single-nucleotide polymorphisms of candidate genes may be associated with genetic susceptibility of the disorders [4, 5].
Tumor suppressor p53 (TP53) gene is regarded as potential guardian of the human genome [6]. TP53 is the most common mutated gene in head and neck cancer, making p53 an appealing target for improving treatment of head and neck cancer by restoring its tumor suppressor action [7, 8]. The rs1042522 G/C polymorphism of TP53 results in the alteration at codon 72 between arginine (Arg) and proline (Pro) and causes the TP53Arg72Pro mutation. This may affect the normal function of the TP53 protein and is implicated in susceptibility to several cancers including OSCC [9,10,11]. A earlier meta-analysis published in BMC Oral Healthreported TP53 codon 72 polymorphism was not associated with OLK susceptibility [12]. Recently, a systematic review and meta-analysis demonstrated that overexpression of p53 immunoprotein was significantly associated with the risk of OPMD malignant progression [13].
Based on the above, we hypothesize that TP53 polymorphism may be associated with the risk of OPMD progression to OSCC. Besides, there are newly published several case–control studies on various types of OPMD, which are suitable to assess the association of TP53 codon 72 polymorphism with the onset of general OPMD. Therefore, the objective of the meta-analysis was to systematically evaluate the relationship between TP53 codon 72 polymorphism and both onset and progression of OPMD based on case–control studies.
Materials and methods
Search strategy and data extraction
A comprehensive literature search was conducted on PubMed, Web of Science, and Medline databases for all relevant publications on the association between TP53 codon 72 polymorphism and OPMD, without any restriction on Feb. 21, 2023. According to the search strategy described in Supplementary Table S1, we used medical subject term (‘‘polymorphism*’’ OR ‘‘gene variant’’) AND (‘‘p53 OR ‘‘TP53’’) AND the synonyms of OPMD in all fields. The inclusion criteria for eligible articles were as follows: (i) human case–control studies; (ii) evaluation of TP53 codon 72 polymorphism and OPMD onset or progression. OPMD progression indicates the polymorphism in OPMD compared with in OSCC; (iii) sufficient genotyping data for the computation of odds ratio (OR) and 95% confidence interval (CI); (iv) histologically confirmed diagnosis of OPMD and OSCC. On the contrary, the exclusion criteria were as follows: (i) not a case–control study; (ii) overlapping or duplicate publications; (iii) no genotype data reported.
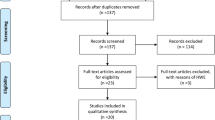
According to the selection criteria, all relevant crude data were extracted from each eligible study independently by two investigators. Inconsistency was discussed until a consensus was obtained with a third investigator. The following information were extracted from each study: first author' name, publication year, country origin, ethnicity, age, sex, tobacco and alcohol use, genotyping methods, number and characteristics of cases and controls, genotype distributions of cases and controls. The case group was OPMD, and control group was healthy individuals or OSCC. Ethical approval and informed consent were not applicable for a meta-analysis. Base on the above process and the flow diagram of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Supplementary Figure S1), 13 eligible case–control studies on TP53 codon 72 polymorphism and OPMD onset or progression were retrieved for detailed evaluation from the literature databases (Table 1) [14,15,16,17,18,19,20,21,22,23,24,25,26].
Statistical analysis
As per the methods described previously [27], the statistical analysis was carried out with the software Review manager 5.4 (The Cochrane Collaboration, Oxford, UK). Hardy–Weinberg equilibrium (HWE) of control group in included studies was measured using Pearson’s goodness-of-fit χ2 test. The strength of association of TP53 codon 72 polymorphism and OPMD onset or progression was determined by calculating odds ratios (ORs) with corresponding 95% credible interval (CI). χ2–based Q-test and I2 statistics were utilized to test statistical heterogeneity, and the Z-test was used to assess the statistical significance of the pooled OR. ORs were pooled according to the fixed-effects model (Mantel–Haenszel model) if heterogeneity was not significant (P > 0.05). Otherwise, the random-effects model (DerSimonian and Laird model) was conducted. Begg's funnel plot and Egger's test were visually examined to evaluate the potential publication bias of the included studies. All tests were used two-sided P value, and the value less than 0.05 was accepted as statistical significance.
Results
Association of TP53 codon 72 polymorphism with OPMD onset
There were 12 eligible case–control studies on TP53 codon 72 polymorphism in OPMD onset, compare to health control (Table 1). A total of 768 OPMD patients and 1173 healthy individuals were identified from six countries including India, China, USA, Argentina, Iran, and Thailand. In the overall analysis, no significantly increased or decreased risk of OPMD onset was observed in the cases for the five genetic models including allele C vs. G [PA (P-value of association test) = 0.39], homozygote CC vs. GG (PA = 0.55), heterozygote GC vs. GG (PA = 0.76), dominant GC + CC vs. GG (PA = 0.27), and recessive CC vs. GG + GC (PA = 0.83). In the stratified analysis by ethnicity, the similar results were observed in five genotype models among Asians and mixed ethnicity. The detailed genotype distributions and forest plots of the five genetic models are depicted in Fig. 1. Begg’s funnel plot showed that there was no obvious evidence for publication bias in five genetic models of TP53 codon 72 polymorphism in OPMD onset (Figure S2). These results indicate that TP53 codon 72 polymorphism may have no significant influence on the risk of OPMD onset.
Association of TP53 codon 72 polymorphism with OPMD progression
There were 8 eligible case–control studies on TP53 codon 72 polymorphism in OPMD progression to OSCC (Table 1). A total of 465 OPMD patients and 775 OSCC ones were identified from four countries including India, China, Argentina, and Iran. In the overall analysis, no significantly increased or decreased risk of OPMD progression to OSCC was observed in the cases for the five genetic models including allele C vs. G [PA = 0.10], homozygote CC vs. GG (PA = 0.19), heterozygote GC vs. GG (PA = 0.44), dominant GC + CC vs. GG (PA = 0.48), and recessive CC vs. GG + GC (PA = 0.48). In the stratified analysis by ethnicity, the similar results were observed in four genotype models among Asians and mixed ethnicity. Constrainedly, an association of TP53 codon 72 polymorphism in allele model with OPMD progression was found (OR, 1.20; 95%CI, 0.98–1.45; P = 0.069) among Asians based on 6 studies. The detailed genotype distributions and forest plots of the five genetic models are illustrated in Fig. 2. Begg’s funnel plot showed that there was no obvious evidence for publication bias in five genetic models of TP53 codon 72 polymorphism in OPMD progression (Figure S3). These results indicate that TP53 codon 72 polymorphism may have no significant influence on the risk of OPMD progression to OSCC.
Discussion
Given the fact that TP53 exhibits diverse behaviors and is involved in various regulatory roles during carcinogenesis, elucidating the role of wild-type and mutated TP53 in the development of OSCC remains a challenge [5,6,7,8]. In an updated meta-analysis, Sun et al. [12] conducted an updated meta-analysis of 17 case–control studies from 16 articles with 3047 cases of OSCC and 3305 health controls, and concluded that there was no significant association between TP53 codon 72 polymorphism and the risk of OSCC in either the Asian or Caucasian population. This data was in agreement with the result from another contemporaneous meta-analysis [28]. Interestingly, Sun et al. [12] performed an additional meta-analysis of 6 case–control studies from 5 articles with 391 cases of OLK and 763 health controls, and also found that no significant association of TP53 codon 72 polymorphism with OLK susceptibility. In this study, we highlighted the potential role of TP53 codon 72 polymorphism in the risk of onset and progression of general OPMD (containing OLK, oral lichen planus, and oral submucous fibrosis) through a pooled-analysis of 13 case–control studies. Overall, the results of this study indicated that TP53 codon 72 polymorphism may not also be associated with the risk of OPMD onset and progression.
Recently, overexpression of p53 immunoprotein was demonstrated to be significantly associated with malignant progression of OPMD [13]. This was inconsistent with the result of TP53 codon 72 polymorphism not associated with OPMD progression in this study, suggesting p53 overexpression in OPMD progression could be not influenced by TP53 codon 72 polymorphism. Tandon et al. examined TP53 codon 72 gene polymorphism and p53 immunoexpression in 6 cases of OLK and 35 cases of OSCC, but they did not investigate the relationship between TP53 polymorphism and p53 immunoexpression in two groups. Zhang et al. [29] reported that p53 protein expression was identified to be affected by TP53 codon 72 polymorphism in low rectal cancer. Dastjerdi MN [30] reported that TP53 codon 72 polymorphism may be correlated with p53 overexpression and increased risk for colorectal cancer. Al-Dhaheri et al. [31] reported that p53 overexpression in the progression towards malignancy of preneoplastic and neoplastic rat mammary glands associated with TP53 polymorphism; while Rybárová et al. [32] reported that no statistically significant difference was found between TP53 codon 72 polymorphism and p53 protein expression in human breast cancer. These variations in results might be possible due to organ specificity and species differences.
Meta-analysis allows stronger quantitative synthesis for identifying some models of risk markers, and reduces the limitations of the relatively small sample size and sampling bias of individual studies [12, 28]. Although the efforts in performing a comprehensive analysis, certain limitations need to be addressed in this study. First, the number of eligible studies available with the pooled sample size of most studies was small and in both overall and subgroup analyses; and it is possible that some relevant studies in some localized databases were missed. Secondly, the effect of the confounding ingredients in gene-environment exposures and lifestyle habits interactions such as environmental factors, and tobacco and alcohol use, were not estimated in the current study due to data available limitation (Table 1). Thirdly, the results were of heterogeneity in some genetic models, possible due to role of various factors such as geographic distribution and racial differences on various predisposing factors involving lifestyle habits. Therefore, to obtain a more accurate results of TP53 codon 72 polymorphism on OPMD onset and progression, additional well-designed studies with larger sample sizes and diverse ethnicities are warranted to validate the associations.
In summary, this is the first pooled-analysis to investigate the association between TP53 codon 72 polymorphism and OPMD onset and progression, suggesting that TP53 polymorphism may not act as genetic factor for the risk of this disease. Combined with the conclusion by a systematic review and meta-analysis [13], we put forward a new opinion that TP53 genetic typing cloud not influence p53 protein expression in OPMD. Further studies are needed to consolidate this opinion.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
References
Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021;27:1862–80.
Farah CS. Molecular, genomic and mutational landscape of oral leukoplakia. Oral Dis. 2021;27(4):803–12.
Singh RD, Patel KA, Patel JB, Patel PS. Alterations in p53 Influence hTERT, VEGF and MMPs expression in oral cancer patients. Asian Pac J Cancer Prev. 2022;23(9):3141–9.
Shridhar K, Aggarwal A, Walia GK, Gulati S, Geetha AV, Prabhakaran D, et al. Single nucleotide polymorphisms as markers of genetic susceptibility for oral potentially malignant disorders risk: Review of evidence to date. Oral Oncol. 2016;61:146–51.
Yasasve M, Manjusha M, Saravanan M. Polymorphism in pro-inflammatory cytokines and their genetic susceptibility towards oral precancerous lesions and oral cancer. Oral Oncol. 2022;133:106027.
Selvaraj J, Yasothkumar D, Vishnu Priya V, Raj AT, Babu SD, Patil S. Development and tumorigenic potential of TP53: a therapeutic target for head and neck squamous cell carcinoma. Oral Oncol. 2022;130:105922.
Adderley H, Rack S, Hapuarachi B, Feeney L, Morgan D, Hussell T, et al. The utility of TP53 and PIK3CA mutations as prognostic biomarkers in salivary adenoid cystic carcinoma. Oral Oncol. 2021;113:105095.
Kobayashi K, Yoshimoto S, Ando M, Matsumoto F, Murakami N, Omura G, et al. Full-coverage TP53 deep sequencing of recurrent head and neck squamous cell carcinoma facilitates prognostic assessment after recurrence. Oral Oncol. 2021;113:105091.
Li B, Li H, Zhang L, Ren T, Meng J. Expression analysis of human glioma susceptibility gene and P53 in human glioma and its clinical significance based on bioinformatics. Ann Transl Med. 2023;11(2):53.
Fan Z, Zhang Q, Feng L, Wang L, Zhou X, Han J, et al. Genomic landscape and prognosis of patients with TP53-mutated non-small cell lung cancer. Ann Transl Med. 2022;10(4):188.
Liu R, Sun K, Wang Y, Jiang Y, Kang J, Ma H. The effects of proliferating cell nuclear antigen and p53 in patients with oral squamous cell carcinoma: a systematic review and meta-analysis. Ann Transl Med. 2021;9(23):1739.
Sun Z, Gao W, Cui JT. Effect of TP53 rs1042522 on the susceptibility of patients to oral squamous cell carcinoma and oral leukoplakia: a meta-analysis. BMC Oral Health. 2018;18(1):143.
Ramos-García P, González-Moles MÁ, Warnakulasuriya S. Significance of p53 overexpression in the prediction of the malignant transformation risk of oral potentially malignant disorders: a systematic review and meta-analysis. Oral Oncol. 2022;126:105734.
Mitra S, Sikdar N, Misra C, Gupta S, Paul RR, Roy B, et al. Risk assessment of p53 genotypes and haplotypes in tobacco-associated leukoplakia and oral cancer patients from eastern India. Int J Cancer. 2005;117(5):786–93.
Misra C, Majumder M, Bajaj S, Ghosh S, Roy B, Roychoudhury S. Polymorphisms at p53, p73, and MDM2 loci modulate the risk of tobacco associated leukoplakia and oral cancer. Mol Carcinog. 2009;48(9):790–800.
Lin YC, Huang HI, Wang LH, Tsai CC, Lung O, Dai CY, et al. Polymorphisms of COX-2 -765G>C and p53 codon 72 and risks of oral squamous cell carcinoma in a Taiwan population. Oral Oncol. 2008;44(8):798–804.
Ye Y, Lippman SM, Lee JJ, Chen M, Frazier ML, Spitz MR, et al. Genetic variations in cell-cycle pathway and the risk of oral premalignant lesions. Cancer. 2008;113(9):2488–95.
Ghabanchi J, Fattahi MJ, Mardani M, Tadbir AA, Paydar AA. Polymorphism of tumor protein p53 codon 72 showed no association with oral lichen planus in Shiraz. Iran J Craniofac Surg. 2009;20(6):2168–70.
Yanatatsaneeji P, Kitkumthorn N, Dhammawipark C, Rabalert J, Patel V, Mutirangura A. Codon72 polymorphism in the p53 tumor suppressor gene in oral lichen planus lesions in a Thai population. Asian Pac J Cancer Prev. 2010;11(4):1137–41.
Sikka S, Sikka P. Association of Human Papilloma Virus 16 infection and p53 polymorphism among tobacco using oral leukoplakia patients: a Clinicopathologic and genotypic study. Int J Prev Med. 2014;5(4):430–8.
Zarate AM, Don J, Secchi D, Carrica A, Galindez Costa F, et al. Study of the TP53 codon 72 polymorphism in oral cancer and oral potentially malignant disorders in Argentine patients. Tumour Biol. 2017;39(5):1010428317699113.
Ramya AS, Majumdar S, Babu TM, Uppala D, Srinivas B, Rao AK. Expression of human papillomavirus DNA and p53 polymorphisms through polymerase chain reaction in normal mucosa and oral leukoplakia individuals with deleterious oral habits. Int J Appl Basic Med Res. 2017;7(2):134–8.
Tandon N, Srivastava AN, Fatima N, Raza ST, Kumar V. p53 codon 72 gene polymorphism studies and p53 expression by immunohistochemistry in oral lesions as risk factor for malignancy. Int J Appl Basic Med Res. 2017;7(4):243–6.
Tabatabaei SH, Sheikhha MH, Karbasi MHA, Zarmehi S, Hoseini M. Evaluation of polymorphism of P53 protein codon 72 in oral lichen planus by PCR technique. J Dent Res Dent Clin Dent Prospects. 2018;12(4):245–51.
Hallikeri K, Burde K, Anehosur V, Kulkarni BB, Hiremath SV. p53 polymorphism and association of human papillomavirus in oral submucous fibrosis and oral squamous cell carcinoma: a case-control study. J Oral Maxillofac Pathol. 2019;23(1):97–103.
Galíndez MF, Carrica A, Zarate AM, Secchi D, Don J, Barra JL, et al. DNA repair, NFKβ, and TP53 polymorphisms associated with potentially malignant disorders and oral squamous cell carcinoma in Argentine patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(3):339–46.
Liu W, Li M, Zhang X, Zhou Z, Shen Z, Shen X. Association of polymorphisms in Th1/Th2-related cytokines (IFN-γ, TGFβ1, IL-1β, IL-2, IL-4, IL-18) with oral lichen planus: a pooled analysis of case-control studies. J Dent Sci. 2023;18(2):560–6.
Lin YM, Shao J, Yin XH, Huang C, Jia XW, Yuan YD, et al. Meta-analysis results on the association between TP53 codon 72 polymorphism with the susceptibility to oral cancer. Front Physiol. 2018;9:1014.
Zhang G, Xu Q, Wang Z, Sun L, Lv Z, Liu J, et al. p53 protein expression affected by TP53 polymorphism is associated with the biological behavior and prognosis of low rectal cancer. Oncol Lett. 2019;18(6):6807–21.
Dastjerdi MN. TP53 codon 72 polymorphism and P53 protein expression in colorectal cancer specimens in Isfahan. Acta Med Iran. 2011;49(2):71–7.
Al-Dhaheri W, Hassouna I, Karam SM. Genetic polymorphisms and protein expression of P53 and BRCA1 in preneoplastic and neoplastic rat mammary glands. Oncol Rep. 2018;39(5):2193–200.
Rybárová S, Vecanová J, Hodorová I, Mihalik J, Čižmáriková M, Mojžiš J, et al. Association between polymorphisms of XRCC1, p53 and MDR1 genes, the expression of their protein products and prognostic significance in human breast cancer. Med Sci Monit. 2011;17(12):BR354-63.
Acknowledgements
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China (82072980), and Innovative Research Team of High-Level Local Universities in Shanghai (SSMU-ZLCX20212300).
Author information
Authors and Affiliations
Contributions
XY and QZ designed the study. HL, XZ and YL extracted, analyzed, and interpreted the data. HL and YL drafted the manuscript. HL revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Search strategy in literature database.
Additional file 2: Figure S1.
Flow diagram of the study selection process. Figure S2. Begg’s Funnel plots of association between TP53 codon 72 polymorphism with OPMD onset in (A) allele model, (B) heterozygote model, (C) homozygote model, (D) dominant model, (E) recessive model. Figure S3. Begg’s Funnel plots of association between TP53 codon 72 polymorphism with OPMD progression in (A) allele model, (B) heterozygote model, (C) homozygote model, (D) dominant model, (E) recessive model.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, H., Liu, Y., Zhou, S. et al. Systematic evaluation of TP53 codon 72 polymorphism associated with onset and progression of oral potentially malignant disorders. BMC Oral Health 23, 659 (2023). https://doi.org/10.1186/s12903-023-03316-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-03316-0