Abstract
Background
The aim of this systematic review (SR) was to evaluate the clinical efficacy of different adjunctive methods/therapies to the non-surgical treatment (NST) of peri-implantitis.
Materials and methods
The protocol of the review was registered in PROSPERO database (CRD42022339709) and was designed according to PRISMA statement. Electronic and hand searches were performed to identify randomized clinical trials (RCTs) comparing non-surgical treatment of peri-implantitis alone versus NST plus any adjunctive method/treatment. The primary outcome was probing pocket depth (PPD) reduction.
Results
Sixteen RCTs were included. Only 2 out of 1189 implants were lost and follow-up ranged from 3 to 12 months. PPD reduction across the studies varied from 0.17 to 3.1 mm, while defect resolution from 5.3% to 57.1%. Systemic antimicrobials were associated to higher PPD reduction (1.56 mm; [95% CI 0.24 to 2.89]; p = 0.02) with high heterogeneity, and treatment success (OR = 3.23; [95% CI 1.17 to 8.94]; p = 0.02), compared to NST alone. No differences were found with adjunctive local antimicrobials and lasers for PPD and bleeding on probing (BoP) reduction.
Conclusions
Non-surgical treatment with or without adjunctive methods may reduce PPD and BoP even if complete resolution of the pocket is unpredictable. Among possible adjunctive methods, only systemic antibiotics seems to provide further benefits, but their usage should be considered with caution.
Similar content being viewed by others
Introduction
Peri-implantitis is a plaque-associated disease occurring around dental implants, characterized by inflammation of the peri-implant mucosa and progressive bone loss [1, 2]. Peri‐implantitis exhibits clinical signs of inflammation (i.e., bleeding on probing [BoP] and/or suppuration), increased probing pocket depth [PPD] and/or recession of the mucosal margin in addition to progressive loss of supporting bone [3].
It is suggested that peri-implantitis progresses in a non-linear, accelerating pattern and that the onset occurs, usually, within 3 years of function [4, 5]. History of periodontitis, poor plaque control, and irregular supportive peri-implant care represent the main risk factors for peri-implantitis [6, 7]. The role of other risk factors remains unclear [8].
It is difficult to define the global prevalence of peri-implantitis, mostly due to the wide range of case definitions used across studies [9]. The available evidence shows that prevalence of periimplantitis ranges between 15 and 56% [4, 8, 10]. Recent data showed an implant loss due to peri-implantitis up to 13.6% at the patient level and up to 8.3% at the implant level [10, 11].
Treatment of peri-implantitis remains challenging for the clinicians. A recent study with 5 years follow-up reported 44% recurrence/progression of peri-implantitis, and 27% implant loss also after the treatment. Residual PPD ≥ 6 mm was the main risk factor for recurrence and progression of peri-implantitis, highlighting the need for a proper intervention in case of peri-implantitis [12].
Both non-surgical and surgical interventions were proposed to treat peri-implantitis [13]. However, the treatment should start from the infection control procedures [14]. The decontamination of the implant surface is more challenging and unpredictable compared to the treatment on natural teeth [15]. On this way, the adjunctive use of lasers [16], systemic or local antibiotics [17] and antimicrobial photodynamic therapy (aPDT) [18], have been proposed and investigated in both pre-clinical and clinical studies in order to increase implant surface decontamination.
Therefore, the aim of this systematic review (SR) was to evaluate the clinical efficacy of different adjunctive methods/therapies to the non-surgical treatment (NST) of peri-implantitis.
Materials and methods
This SR was reported in accordance with the PRISMA statement [19]. The review protocol was registered in PROSPERO database (CRD42022339709).
Focus question
PICO method and the guidelines of the Center for Evidence-Based Medicine (University of Oxford, Oxford, UK) [20] were used, and the focused question was: “In patients with peri-implantitis, what is the additional benefit of adding adjunctive methods to non-surgical therapy compared with to non-surgical therapy alone?”.
Population
Adult patients, affected by peri-implantitis, not affected by systemic diseases (i.e., diabetes, autoimmune diseases).
Intervention
Non-surgical implant surface debridement.
Comparison
Any adjunctive treatment to the non-surgical implant surface debridement.
Outcomes
The primary outcome was PPD reduction. Secondary outcomes were: relative clinical attachment level (RAL) gain, marginal bone level (MBL) changes, BoP reduction, composite outcome for treatment success/resolution, Implant Survival.
Eligibility criteria
-
Only randomized clinical trials (RCTs) treating at least 5 patients per group and reporting PPD reduction in millimeters at final follow-up visit were considered.
-
Each patient had at least one dental implant suffering from peri-implantitis, treated by means of non-surgical debridement with or without adjunctive methods.
-
A diagnosis of peri-implantitis in accordance with the 2017 classification [3]. Studies published or designed before 2017 were included only if the diagnosis of peri-implantitis was based on the same criteria of the 2017 classification.
-
Minimum follow-up was 3 months.
-
Only English language articles were considered.
Information sources and search strategy
Three on-line databases (PUBMED, EMBASE, and COCHRANE) were used. The last search was on October 20, 2022. (For detail information on the search strategy see Additional file 1).
Following journals were hand searched from July 2012 to October 2022, Clinical Implant Dentistry and Related Research, Journal of Clinical Periodontology, Journal of Periodontology, Journal of Periodontal Research, Journal of Dental Research, Clinical Oral Implant Research. The references list of the included studies and other relevant SRs on the topic were also examined for additional papers.
Selection process
Studies' eligibility was independently assessed by two reviewers (C.R. and D.S.). Records were screened by title and abstract after duplicates removal, then the eligible papers were evaluated in full text. In case of disagreement, a third reviewer (L.B.) was involved for the final decision. The level of agreement between the two examiners was evaluated using Cohen's kappa coefficient (k).
Data collection process and data items
A customized table was used by two reviewers (C.R. and D.S.) to retrieve information and data from the included studies. For each study, the following information were considered: authors, year of publication, country, definition of peri-implantitis, follow-up, n. of patients, n. of implants, type of treatment, diagnostic criteria for peri-implantitis and outcomes of interest (PPD Reduction, RAL Gain, MBL Changes, BoP changes, treatment success/resolution, Implant Survival). In case of missing or unclear data authors were contacted to clarify any doubt.
Risk of bias assessment
The risk of bias of the included studies was evaluated using the Cochrane Collaboration’s Tool RoB 2.0 [21]. Briefly, 5 domains (Risk of bias arising from the randomization process, Risk of bias due to deviations from the intended interventions, Risk of bias due to missing outcome data, Risk of bias in measurement of the outcome, Risk of bias in selection of the reported result) were evaluated [21]. Each included study was rated as:
-
A.
Low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met.
-
B.
Some concerns risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were partly met.
-
C.
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
Effect measures and synthesis methods
Studies were initially grouped by characteristics and according to the type of adjunctive treatment. A meta-analysis was performed in case of at least two studies of similar design. Differences between groups were expressed as weighted mean differences (WMD) and 95% confidence interval (CI) for continuous outcomes and odds ratio (OR) and 95% CI for dichotomous outcomes. Standard deviation from the mean differences were calculated when not available, according to Cochrane Handbook for Systematic Reviews of Interventions [22]. Random-effect model was used, and the variables were registered at implant level. The arms were weighted according to the treated sample size [23]. For studies with multiple intervention groups, groups were combined to create a single pair-wise comparison, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions [22].
The heterogeneity was assessed by means of the I2 statistics (0%–40% low heterogeneity, 30%–60% moderate heterogeneity, 50%–90% substantial heterogeneity and 75%–100% considerable heterogeneity) [24].
The statistical analyses were carried out using the RevMan software version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and the STATA software package (version 15.0).
Results
Study selection
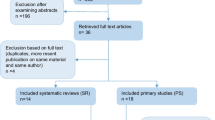
The search strategy identified 320 potentially eligible articles. After screening of titles and/or abstracts, 43 articles were selected for full text evaluation and 12 additional papers were retrieved from hand search. Thus, 55 articles were subjected to the eligibility process. Finally, 16 articles were included in the review (Fig. 1) [17, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. For details on excluded full-text articles see Additional file 2. Cohen's kappa value for the global inter-reviewer agreement was 0.83.
Study characteristics and results of individual studies
RCTs included in this SR were published between 2012 and 2022. For additional information about country, setting and funding, see Additional file 3.
Follow-up was 3 months in four studies [33, 35, 38, 39], 6 months in six studies [26, 27, 29, 31, 32, 36], 12 months in two studies [28, 30] and data at 3 and 6 months were reported in 3 studies [25, 34, 37] while one study reported data at 6 and 12 months [17].
Initial supragingival professional hygiene was performed in every study before the treatment. All the papers specified that the patients were given detailed instructions for self-plaque control measures before starting the treatment.
Adjunctive therapies used were local antibacterial agents, systemic antibiotics, lasers, aPDT, probiotics and a desiccant agent. Three studies evaluated the adjunctive effect of systemic antibiotics, but using different drugs or dosage: metronidazole 500 mg 3/die for seven days [17]; metronidazole 400 mg and amoxicillin 500 mg, 3/die for 14 days [28]; metronidazole 250 mg and amoxicillin 375 mg three times a day for 7 days [33].
Local antibacterial agents were investigated in four studies. In particular, two studies tested multiple applications of chlorhexidine chips [26, 27] one tested the application of chloramine [38], and another study evaluated the application of minocycline with or without metronidazole [39]. Moreover, different lasers were tested in five studies [29, 37, 32, 30, 34] and two studies used the aPDT as adjunctive method to the debridement alone [35, 36]. Topical application of a dual-strain Lactobacillus Reuteri probiotic was tested in one RCT [25]. Finally, Merli et al. performed a three arms RCT evaluating both an air-polishing device and a desiccant material [31].
Only two out of 1189 implants with peri-implantitis treated in the studies were lost during the follow-up. PPD reduction was reported in all group testing debridement alone and varied from 0.2 mm to 1.8 mm. For adjunctive therapies, the use of diode laser was associated to the worst PPD reduction of 0.17 mm while the greatest one was 3.1 mm for systemic antibiotics. Ten studies reported data on BoP changes: BoP was reduced from 10 to 50%. Radiographic bone level changes were reported in three studies showing improvement for both control and test group.
Only 6 studies used a composite outcome for treatment success and the definition was homogeneous (absence of PPD ≥ 5 mm [27], absence of 5 mm PPD associated with BoP and no progressive bone loss [17, 31, 33, 34, 39]). None of the included studies reported a 100% resolution rate, with the success ranging between 5.3% and 57.1%.
The main outcomes are summarized in Table 1. Secondary outcomes are reported in Additional file 4.
Definitions of Peri-implantitis
The included RCTs defined peri-implantitis according to the following parameters: PPD, BoP and evidence of radiographic peri-implant bone loss. Although all studies provided a definition of peri-implantitis consistent with the 2017 classification, slight differences were retrieved between the different papers. In order to provide reliable information to the reader, the definitions used by each study for the diagnosis of peri-implantitis were reported in Table 2.
Risk of bias in studies
Out of 16 included studies, 5 were rated at high risk of bias, 4 at unclear risk of bias, and 7 at low risk of bias (Fig. 2).
Risk of Bias of the included studies (RoB 2 tool according to Sterne et al.). Risk of bias legend: R: bias arising from the randomization process; D: bias due to deviations from intended interventions; Mi: bias due to missing outcome data; Me: bias in measurement of the outcome; S: bias in selection of the reported results; O: overall risk of bias. Judgements:
 Low risk of bias;
Low risk of bias;
 High risk of bias;
High risk of bias;
 Some Concerns
Some Concerns
Results of syntheses
Thirteen RCTs were included in the meta-analyses and a total of seven meta-analyses were performed (Figs. 3, 4 and 5).
The use of systemic antimicrobials was the only adjunctive method which provided a statistically significant PPD reduction (1.56 mm; [95% CI 0.24 to 2.89]; p = 0.02), with higher probability of treatment success (OR = 3.23; [95% CI 1.17 to 8.94]; p = 0.02), compared to debridement alone. The adjunctive use of local antimicrobials did not provide additional benefit for PPD reduction (1.03 mm; [95% CI -0.06 to 2.11]; p = 0.06) and BoP reduction (0.6%; [95% CI -0.22 to 1.42]; p = 0.43) compared to debridement alone. Furthermore, the use of Lasers was not associated to PPD (0.28 mm; [95% CI -0.29 to 0.85]; p = 0.33) or BoP reduction (0.52%; [95% CI -0.44 to 1.48]; p = 0.29) and aPDT for PPD reduction (0.28 mm; [95% CI -0.39 to 0.94]; p = 0.41).
The heterogeneity was considerable in 3 meta-analyses: PPD Reduction for Mechanical Debridement (MD) vs MD + local antimicrobials (I2 = 96%; p < 0.05), PPD reduction for MD vs MD + systemic antibiotics (91%; p < 0.05) and BoP Reduction for MD vs local antimicrobials (86%; p < 0.05).
Discussion
Summary of main findings
The findings of the present SR showed that only 2 implants out of 1089 were lost at the final follow-up (3–12 months). However, this should be considered as potential proof of success with caution. In fact, peri-implant health is defined as the absence of inflammation, absence of BoP and no evidence of increased PPD/bone loss following initial healing [40]. When a composite outcome (i.e., minimal PPD, no BoP, no progressive bone loss) was considered to define treatment success after non-surgical treatment, the rate of resolution was low and unpredictable (from 5.3 to 57.1%). Additionally, follow-up was short (3 to 12 months), and no data were available in the long-term. This could be explained by the low-resolution rate after non-surgical treatment and the subsequent need for surgery, which made very difficult to have medium and long-term data on implant survival using non-surgical approaches. Thus, non-surgical treatment associated or not to adjunctive methods cannot be considered predictable for the resolution of peri-implantitis in the long-term, avoiding implant loss.
Nevertheless, non-surgical treatment was associated to reduction of peri-implant pocket depth. All the studies included in this SR reported PPD reduction after implant debridement (alone) that varied from 0.2 mm to 1.8 mm. This heterogeneity in the outcomes may be explained by some elements including different conditions at baseline and diverse ability of operators. Even if data are limited, pocket-related response may be also expected, since higher PPD reduction at 3 months for ≥ 7 mm pockets (2.78 mm) compared to 4-6 mm pockets (1.24 mm) was described in a trial [2]. Baseline mean PPD values in this SR were heterogeneous (4.14 mm to 8 mm), even though there were no significant differences between groups in the single studies.
Both peri-implant mucositis and peri-implantitis are characterized by tissues inflammation. Even though it is difficult to define the role of the tissue inflammation on the progression from mucositis to peri-implantitis, the evolution of patients affected by peri-implant mucositis was evaluated in a retrospective study [6]. After 5 years, BoP at more than 50% of the sites and PPD ≥ 4 mm at more than 5% of the sites were associated to risk of progression to peri-implantitis. It could be speculated that prolonged tissue inflammation is a main risk factor for progression. In this SR, non-surgical treatment was effective in reducing BoP values between 5.3 to 57.1%. However, results were heterogeneous and residual inflammation was present at majority of treated implants. Thus, non-surgical therapy should be considered unpredictable in reducing BoP at peri-implantitis site.
Agreements and disagreements with other SR and studies
A previous SR with a Bayesian network meta-analysis was performed by Faggion et al. in 2014, including 11 studies [41]. The aim was to compare the clinical effect of various non-surgical peri-implantitis therapies. The authors concluded that the evidence was not sufficient to support the superiority of any treatment. Nevertheless, MD + antibiotics achieved an estimate difference of 0.490 mm for PPD reduction in comparison to debridement alone. Despite the differences in methodology, our results are in agreement with their SR.
Systemic antibiotics determined a significant PPD reduction with a difference of 1.56 mm (p < 0.02) compared to debridement alone in this SR. Similarly, a difference of 1.46 mm favoring adjunctive systemic antibiotics was reported in another SR [42]. Contrary, different results were found in a recent RCT by De Waal et al. [2]. In their RCT, peri-implantitis was treated by means of full-mouth mechanical debridement and air-powder (erythritol powder containing chlorhexidine) in the control group, while test group received adjunctive systemic AMX/MTZ also. After 3 months, clinical conditions improved in both groups, but no significant differences for any outcome were reported. It could be speculated that other adjunctive methods or combinations may reach similar PPD reduction compared to systemic antibiotics.
Nevertheless, it is mandatory to analyze the clinical effectiveness in terms of defect resolution and further need of additional surgery. In our SR, the use of systemic antibiotics determined a threefold increase of treatment success chance.
This result should be interpreted with extreme caution because, looking into the data retrieved from the single studies, the number of diseased implants after non-surgical therapy remained high and there was a consistent heterogeneity among the proposed experimental treatments. Additionally, no data were available in the long-term, since included RTCs had a maximum 1-year follow-up. Within this context, it is very difficult to understand the role of adjunctive systemic antibiotics in reducing the need for surgery. Moreover, it is worth to mention the issue of antibiotic resistance. The subgingival peri-implant pathogens were found to be resistant in vitro to individual concentration of clindamycin, amoxicillin, doxycycline, or metronidazole in 71.7% of the subjects [43]. Finally, all the studies used metronidazole, and its administration for oral infection is not allowed in all countries.
The local application of antimicrobials was associated to a not statistically significant difference in terms of PPD reduction (1.03 mm; [95% CI -0.06 to 2.11]; p = 0.06) compared to debridement alone. The heterogeneity was considerable. Two of the studies included in this meta-analysis were multicenter RCTs and tested repeated applications of chlorhexidine chips. Another trial tested the efficacy of minocycline and/or metronidazole ointments. Our results are similar to those reported in a previous SR on peri-implantitis [41] and in a more recent SR focusing on peri-implant mucositis [44]. Thus, the efficacy of repeated applications of chlorhexidine chips or minocycline and/or metronidazole remains controversial.
This SR failed to demonstrate significant clinical benefit for adjunctive use of lasers for both PPD (0.28 mm; [95% CI -0.29 to 0.85]; p = 0.33) and BoP reduction (0.52%; [95% CI -0.44 to 1.48]; p = 0.29). In accordance with these results, a recent SR found only minimal benefit using lasers in terms of PPD reduction (0.15 mm) after non-surgical therapy [45]. Similarly, a previous SR [46], aiming to evaluate the effectiveness of laser therapy in managing peri-implant mucositis and peri-implantitis, failed to reveal any superiority when laser treatment was performed, alone or as an adjunctive.
A RCT found higher BoP reduction with the adjunctive use of a Er:YAG laser after 6 months, however after 12 months no significant differences were reported [47]. It could be hypothesized that self-performed oral hygiene and adherence to supportive peri-implant therapy may be more important for controlling the bacterial colonization of the peri-implant pocket that the treatment itself.
Although in vitro studies showed that aPDT may be effective in bacterial killing on titanium surfaces [48], clinical improvement in terms of PPD reduction was minimal for aPDT (0.33 mm; [95% CI -0.34 to 1.01]; p = 0.33) in this SR, and this data are in accordance with a recently published SR [49].
It could be hypothesized that, even if aPDT may be effective in bacterial killing having an initial effect, bacterial recolonization of the peri-implant pocket is not prevented especially in case on incomplete resolution [50]. In fact, peri-implantitis resolution was under 50% for adjunctive PDT, lasers or chlorhexidine (CHX) [31, 51, 52]. Contrary, another SR [42] found that adjunctive aPDT therapy led to significant PD reduction over a 6-month period compared to the mechanical debridement alone. However, this conclusion was based upon the analysis of a single study [53] that was not included in our SR. Therefore, the clinical efficacy of adjunctive aPDT remains controversial.
Quality of the evidence and potential limitations in the review process
This SR has several limitations. It included only studies in which the control group was the debridement alone, in order to reduce the potential source of bias and have a clear view of the benefits of adjunctive methods; thus, direct comparisons between different adjunctive therapies were not possible. Furthermore, at the time the included RCTs were performed, there were huge differences in defining peri-implantitis. Different criteria for the diagnosis of peri-implantitis also implies differences in baseline PPD that may have influenced the results of pooled estimates. Unfortunately, subgroup analyses stratified according to baseline PPD were not considered appropriate, due to the paucity of studies included in each meta-analysis.
Finally, even though composite outcomes could be considered appropriate to define the efficacy of the therapy, only short-follow-up data were available and very few studies used composite outcomes (i.e., PPD and BoP and MBL) to report disease resolution. Future RCTs should consider composite outcomes to define disease resolution, in order to improve the understanding of the clinical effectiveness of peri-implantitis methods and therapies.
Another factor that may jeopardize the results of our SR lies in the risk of bias in the included studies. Although every effort was made to include high quality papers, only 7 RCTs were rated at low risk of bias. Future studies should provide a better description of the randomization process and of the allocation concealment, which were frequently omitted.
Finally, another limitation of this SR lies in the fact that half of the included papers did not provide any information about the implant surfaces. Machined implants were treated in one study [28], while six studies treated “moderate rough” surfaces without specifying the implant brand [17, 27, 35,36,37] except for the study from Roccuzzo [34]. The biofilm removal may be difficult due to geometry of the threads, and the presence of irregular rough/porous titanium surface made difficult to obtain the complete elimination of the bacterial biofilm, jeopardizing the clinical results. Future studies should better describe these data, in order to understand how much the implant characteristics influence the clinical results.
Conclusions
Withing the limits of this SR, it can be concluded that:
-
Non-surgical treatment of peri-implantitis, with or without the use adjunctive methods, may reduce PPD and BoP
-
Complete disease resolution is unpredictable to achieve with non-surgical peri-implant therapy.
-
The adjunctive use of systemic antibiotics seems to improve the efficacy of MD, but their use should be considered with caution in routine practice.
-
Minimal benefit was found for the adjunctive use of laser and aPDT, when compared to MD alone.
Implications for practice and future research
The clinical benefit of adjunctive lasers, aPDT and local antimicrobial therapies for NST of peri-implantitis remains unclear and should not be used routinely. Systemic AMX/MTZ may improve PPD reduction and could be considered in specific cases.
Future studies should be designed considering peri-implant disease resolution/health defined as composite outcome (PPD, BOP, MBL) to better understand the role of adjunctive therapy.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- BoP:
-
Bleeding on Probing
- PPD:
-
Pocket Probing Depth
- aPDT:
-
Antimicrobial PhotoDynamic Therapy
- SR:
-
Systematic Review
- NST:
-
Non-Surgical Treatment
- RAL:
-
Relative clinical Attachment Level
- MBL:
-
Marginal Bone Level
- RCT:
-
Randomized Clinical Trial
- WMD:
-
Weighted Mean Differences
- CI:
-
Confidence Interval
- OR:
-
Odds Ratio
- MD:
-
Mechanical Debridement
- CHX:
-
Chlorhexidine
References
Berglundh T, Armitage G, et al. Periimplant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45(Suppl 20):S286–91.
De Waal Y, Vangsted T, Van WA. Systemic antibiotic therapy as an adjunct to non-surgical peri-implantitis treatment: a single-blind RCT. J Clin Periodontol. 2021;48(7):996–1006.
Schwarz F, Derks J, Monje A, Wang H. Peri-implantitis. J Clin Periodontol. 2018;45(Suppl 20):S246–66.
Derks J, Schaller D, Håkansson J, Wennström J, Tomasi C, Berglundh T. Peri-implantitis - onset and pattern of progression. J Clin Periodontol. 2016;43(4):383–8.
Zitzmann N, Berglundh T, Ericsson I, Lindhe J. Spontaneous progression of experimentally induced periimplantitis. J Clin Periodontol. 2004;31(10):845–9.
Costa F, Takenaka-Martinez S, Cota L, Ferreira S, Silva G, Costa J. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012;39(2):173–81.
Karoussis I, Salvi G, Heitz-Mayfield L, Bragger U, Hammerle C, Lang N. Long-term implant prognosis in patients with and without a history of chronic periodontitis: a 10-year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res. 2003;14:329–39.
Romandini M, Lima C, Pedrinaci I, Araoz A, Soldini M, Sanz M. Prevalence and risk/protective indicators of peri-implant diseases: a university-representative cross-sectional study. Clin Oral Implants Res. 2021;32(1):112–22.
Salvi G, Cosgarea R, Sculean A. Prevalence and mechanisms of peri-implant diseases. J Dent Res. 2017;96(1):31–7.
Derks J, Tomasi C. Peri-implant health and disease. a systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158–71.
Doornewaard R, Jacquet W, Cosyn J, De BH. How do peri-implant biologic parameters correspond with implant survival and peri-implantitis? A critical review. Clin Oral Implants Res. 2018;29(Suppl 18):100–23.
Carcuac O, Derks J, Abrahamsson I, Wennström J, Berglundh T. Risk for recurrence of disease following surgical therapy of peri-implantitis-A prospective longitudinal study. Clin Oral Implants Res. 2020;31(11):1072–7.
Barootchi S, Wang H. Peri-implant diseases: Current understanding and management. Int J Oral Implantol (Berl). 2021;14(3):263–82.
Heitz-Mayfield L, Salvi G, Botticelli D, et al. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22(3):237–41.
Wong R, Hiyari S, Yaghsezian A, et al. Comparing the healing potential of late-stage periodontitis and Peri-Implantitis. J Oral Implantol. 2017;43(6):437–45.
Schwarz F, Nuesry E, Bieling K, Herten M, Becker J. Influence of an erbium, chromium-doped yttrium, scandium, gallium, and garnet (Er, Cr:YSGG) laser on the reestablishment of the biocompatibility of contaminated titanium implant surfaces. J Periodontol. 2006;77(11):1820–7.
Blanco C, Pico A, Dopico J, Gándara P, Blanco J, Liñares A. Adjunctive benefits of systemic metronidazole on non-surgical treatment of peri-implantitis. A randomized placebo-controlled clinical trial. J Clin Periodontol. 2022;49(1):15–27.
Romanos G, Nentwig G. Regenerative therapy of deep peri-implant infrabony defects after CO2 laser implant surface decontamination. Int J Periodontics Restorative Dent. 2008;28(3):245–55.
Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71.
Miller S, Forrest J. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Based Dent Prac. 2001;1:136–41.
Sterne J, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. 2022.
Eldridge S, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol. 2006;35(5):1292–300.
Higgins J, Green S. Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration. 2011.
Laleman I, Pauwels M, Quirynen M, Teughels W. The Usage of a Lactobacilli Probiotic in the Non-Surgical Therapy of Peri-Implantitis: A Randomized Pilot Study. Clin Oral Implant Res. 2020;31(1):84–92.
Machtei E, Romanos G, Kang P, et al. Repeated delivery of chlorhexidine chips for the treatment of peri-implantitis: a multicenter, randomized, comparative clinical trial. J Periodontol. 2021;92(1):11–20.
Machtei E, Frankenthal S, Levi G, et al. Treatment of peri-implantitis using multiple applications of chlorhexidine chips: a double-blind, randomized multi-centre clinical trial. J Clin Periodontol. 2012;39(12):1198–205.
Shibli J, Ferrari D, Siroma R, Figueiredo L, Faveri M, Feres M. Microbiological and clinical effects of adjunctive systemic metronidazole and amoxicillin in the non-surgical treatment of peri-implantitis: 1 year follow-up. Braz Oral Res. 2019;33(suppl 1):e080.
Arısan V, Karabuda Z, Arıcı S, Topçuoğlu N, Külekçi G. A randomized clinical trial of an adjunct diode laser application for the nonsurgical treatment of peri-implantitis. Photomed Laser Surg. 2015;33(11):547–54.
Strauss G, Goteiner D, Murawski K, Singer S, Drew H, Sullivan A. Laser-Assisted Therapy for the Treatment of Peri-implantitis. Part I. Clinical Outcomes. Int J Periodontics Restorative Dent. 2021;41(4):563–8.
Merli M, Bernardelli F, Giulianelli E, et al. Short-term comparison of two non-surgical treatment modalities of peri-implantitis: Clinical and microbiological outcomes in a two-factorial randomized controlled trial. J Clin Periodontol. 2020;47(10):1268–80.
Alpaslan Y, Talmac A, Keskin S, Akbal D, Altindal D, Ertugrul A. Erbium, chromium-doped: yttrium, scandium, gallium, garnet and diode lasers in the treatment of peri-implantitis: clinical and biochemical outcomes in a randomized-controlled clinical trial. Lasers Med Sci. 2022;37(1):665–74.
Polymeri A, van der Horst J, Anssari D, Wismeijer D, Loos B, Laine M. Non-surgical peri-implantitis treatment with or without systemic antibiotics: a randomized controlled clinical trial. Clin Oral Implants Res. 2022;33(5):548–57.
Roccuzzo A, Klossner S, Stähli A, et al. Non-surgical mechanical therapy of peri-implantitis with or without repeated adjunctive diode laser application. A 6-month double-blinded randomized clinical trial. Clin Oral Implants Res. 2022;33(9):900–12.
Al-Askar M, Abdullatif F, Alshihri A, et al. Comparison of photobiomodulation and photodynamic therapy as adjuncts to mechanical debridement for the treatment of peri-implantitis. Technol Health Care. 2022;30(2):389–98.
Alqahtani F, Alqhtani N, Alkhtani F, Divakar D, Al-Kheraif A, Javed F. Efficacy of mechanical debridement with and without adjunct antimicrobial photodynamic therapy in the treatment of peri-implantitis among moderate cigarette-smokers and waterpipe-users. Photodiagnosis Photodyn Ther. 2019;28:153–8.
Alqahtani F, Alqahtani N, Celur S, Divakar D, Al-Kheraif A, Alkhtani F. Efficacy of Nonsurgical Mechanical Debridement With and Without adjunct low-level laser therapy in the treatment of Peri-Implantitis: a randomized controlled trial. J Oral Implantol. 2020;46(5):526–31.
Roos-Jansåker A, Almhöjd U, Jansson H. Treatment of peri-implantitis: clinical outcome of chloramine as an adjunctive to non-surgical therapy, a randomized clinical trial. Clin Oral Implants Res. 2017;28(1):43–8.
Park S, Song Y, Cha J, et al. Adjunctive use of metronidazole-minocycline ointment in the nonsurgical treatment of peri-implantitis: A multicenter randomized controlled trial. Clin Implant Dent Relat Res. 2021;23(4):543–54.
Renvert S, Persson G, Pirih F, Camargo P. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Clin Periodontol. 2018;45(Suppl 20):S278–85.
Faggion C, Listl S, Frühauf N, Chang H, Tu Y. A systematic review and Bayesian network meta-analysis of randomized clinical trials on non-surgical treatments for peri-implantitis. J Clin Periodontol. 2014;41(10):1015–25.
Ramanauskaite A, Fretwurst T, Schwarz F. Efficacy of alternative or adjunctive measures to conventional non-surgical and surgical treatment of peri-implant mucositis and peri-implantitis: a systematic review and meta-analysis. Int J Implant Dent. 2021;7(1):112.
Rams T, Degener J, van WA. Antibiotic resistance in human peri-implantitis microbiota. Clin Oral Implants Res. 2014;25(1):82–90.
Barootchi S, Ravidà A, Tavelli L, Wang H. Nonsurgical treatment for peri-implant mucositis: a systematic review and meta-analysis. Int J Oral Implantol (Berl). 2020;13(2):123–39.
Lin G, Suárez LDAF, Wang H. Laser therapy for treatment of peri-implant mucositis and peri-implantitis: An American Academy of Periodontology best evidence review. J Periodontol. 2018;89(7):766–82.
Kotsakis G, Konstantinidis I, Karoussis I, Ma X, Chu H. Systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis. J Periodontol. 2014;85(9):1203–13.
Schwarz F, Bieling K, Bonsmann M, Latz T, Becker J. Nonsurgical treatment of moderate and advanced periimplantitis lesions: a controlled clinical study. Clin Oral Investig. 2006;10(4):279–88.
Azizi B, Budimir A, Mehmeti B, et al. Antimicrobial efficacy of photodynamic therapy and light-activated disinfection against bacterial species on titanium dental implants. Int J Oral Maxillofac Implants. 2018;33(4):831–7.
Chambrone L, Wang H, Romanos G. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J Periodontol. 2018;89(7):783–803.
Clever K, Schlegel KA, Kniha H, et al. Experimental peri-implant mucositis around titanium and zirconia implants in comparison to a natural tooth: part 2-clinical and microbiological parameters. Int J Oral Maxillofac Surg. 2019;48(4):560–5.
Renvert S, et al. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: a randomized clinical trial. J Clin Periodontol. 2011;38(1):65–73.
Schär D, et al. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res. 2013;24(1):104–10.
Wang H, Li W, Zhang D, Li W, Wang Z. Adjunctive photodynamic therapy improves the outcomes of peri-implantitis: a randomized controlled trial. Aust Dent J. 2019;64(3):256–62.
Acknowledgements
N/A
Funding
This paper was written without any financial support or funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.B, C.R., R.C. and F.C.; data curation, L.B, C.R., and D.S.; methodology, L.B., LC; formal analysis, C.R, L.B.; investigation, C.R., D.S; writing—original draft preparation, C.R., D.S. and L.B.; writing—review and editing, L.B., L.S, R.C., F.C.; supervision, F.C, LC.; project administration, F.C., L.B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Consent for publication
N/A.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barbato, L., Cavalcanti, R., Rupe, C. et al. Clinical efficacy of adjunctive methods for the non-surgical treatment of peri-implantitis: a systematic review and meta-analysis. BMC Oral Health 23, 375 (2023). https://doi.org/10.1186/s12903-023-03058-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-03058-z