Abstract
Background
Deterioration in shear bond strength has been reported after immediate bracket bonding following hydrogen peroxide bleaching. This study compared the effectiveness of three antioxidant agents, namely, alpha-tocopherol, green tea extract, and sodium ascorbate, in reversing the bleaching effect and as possible alternatives to delayed bonding.
Methods
A total of 105 extracted human premolars were arbitrarily assigned to 7 groups (n = 15 each), including group 1 as the unbleached control group and six experimental groups, which were bleached with 40% hydrogen peroxide in three sessions of 15 min each. In experimental group 2, bonding was performed immediately after bleaching, whereas in groups 3 and 4, bonding was delayed for 1 and 2 weeks, respectively; meanwhile, the specimens were immersed in artificial saliva at 37 °C. Groups 5, 6, and 7 were treated immediately after bleaching with 10% of alpha-tocopherol, green tea extract, and sodium ascorbate solutions, respectively, for 15 min. Specimens were processed using 500 thermal cycles between 5 and 55 °C, with a dwell time of 30 s after 24 h of bracket bonding, and then tested for shear bond strength. The adhesive remnant index was examined to evaluate fracture mode. One-way analysis of variance, Kruskal–Wallis H, and post hoc Tukey’s honestly significant difference tests were used to compare the data. Significant results were subjected to pairwise comparisons with Bonferroni’s correction-adjusted of p values ≤ 0.050.
Results
Shear bond strength was significantly lower (p < 0.001) in the immediate bonding and 1-week delay groups than in the control group. However, no significant difference was detected among the 2-week delay, antioxidant-treated, and control groups (p > 0.05).
Conclusions
Application of 10% alpha-tocopherol, green tea extract, or sodium ascorbate for 15 min could restore shear bond strength after 40% hydrogen peroxide bleaching as an alternative to delay in bracket bonding.
Similar content being viewed by others
Background
In 1884, Harlan [1] introduced the concept of teeth bleaching using hydrogen peroxide (HP). Since then, considering the growing demand for aesthetic procedures, HP bleaching has been used as a minimally invasive approach for in-office management of tooth discoloration with immediately visible results.
HP bleaching releases free radicals of hydroxyl, per-hydroxyl, peroxide, singlet oxygen, and superoxide anions that target the double bond of the chromogenic complex structures causing tooth discoloration, thereby eventually reducing the discoloration over time [2, 3]. Immediate amelioration in tooth color could raise the patients’ ambitions of having their teeth aligned. However, deterioration in the shear bond strength (SBS) of orthodontic brackets after the application of HP was reported because the residuals of free radicals in tooth structures hinder complete resin polymerization by binding to the polymer chains in adhesive resin, Consequently, the elongation of the polymer chain is terminated and the SBS of brackets to tooth structures decreases [4].
Fortunately, the reduction in SBS appears to be transient, and a recovery period after bleaching, ranging from 24 h to 2 weeks or more, has been recommended [5,6,7,8]. Some strategies for avoiding delays in bonding involve treating enamel with alcohol, adhesives with organic solvents, or removing the superficial enamel layer [9,10,11]. Moreover, the application of different antioxidant agents after HP bleaching instantaneously boosts SBS. These antioxidants act as scavengers of residual free radicals by donating their free electrons, thereby ending the electron-taking reaction of the bleaching process and promoting complete resin polymerization. Several researchers have hypothesized that the immediate application of antioxidants, including ascorbic acid, sodium ascorbate (SA), proanthocyanidins, grape seed, cranberry, extracts of pine bark, alpha-tocopherol (α-T), sodium bicarbonate, aloe vera, and green tea (GT) extracts, would reverse the reduction in SBS [10, 12,13,14,15].
α-T, an active component of the vitamin E complex, is the most effective lipid-soluble antioxidant in nature [15, 16]. GT, which comprises flavanols or catechins that help scavenge excess free radicals, has been used as a promising antioxidant [16, 17]. Ascorbic acid (vitamin C) is believed to improve bond strength; however, the low pH of ascorbic acid (approximately 4.0) can lead to over-conditioning of the enamel surface. Therefore, SA, the neutral salt of ascorbic acid (pH of approximately 7.0), is more applicable intraorally [14, 18, 19].
This study aimed to compare the in vitro effectiveness of the immediate application of three antioxidant agents (10% α-T, 10% GT, and 10% SA) on SBS and investigate whether such treatment could be considered an alternative to delayed bonding of orthodontic brackets after 40% HP bleaching.
Methods
The ethical committee of Mansoura University Dental Faculty, Mansoura, Egypt, provided institutional approval for this study (#A08030821).
Specimen preparation
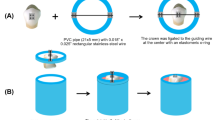
A total of 105 human premolars extracted for orthodontic treatment were collected. Teeth had a sound enamel buccal surface; were devoid of caries, cracks, fracture, fluorosis, restoration, or any developmental anomalies; and had not been treated previously with any chemicals. Debris was removed from the collected teeth using a manual periodontal curette. Then, the teeth were polished using a low-speed handpiece (APPLEDENTAL, Guangdong JINME Medical Technology Co. Ltd., Foshan, China) with a coolant system and oil-free, fluoride-free polishing paste (PROPHY PASTE; PSP Dental Co. Ltd., Kent, UK). Subsequently, the teeth were preserved at room temperature in distilled water, which was changed daily, until usage. Thereafter, each tooth was adjusted within a 15 X 15-mm self-curing acrylic resin block (Acrostone, Cairo, Egypt), embedding the root up to the cemento–enamel junction in which each tooth was manually placed in a central position so that the buccal surface was set perpendicular to the block base.
The specimens were arbitrarily assigned to seven groups (n = 15 for each; Fig. 1), including a control group and six experimental groups. Specimens in group 1 (control group) were not subjected to bleaching or antioxidant application. They were immediately bonded to brackets and preserved at 37 °C in artificial saliva, which was prepared using 19 mg/L MgCl2.6H2O, 544 mg/L KH2PO4, 2240 mg/L KCl, and 103 mg/L of CaCl2 (pH ~ 7.0) at the Analytical Chemistry Department, Faculty of Pharmacy, Mansoura University, Mansoura, Egypt [20]. Specimens in all experimental groups (groups 2–7) were subjected to 40% HP bleaching. After bleaching, teeth in group 2 were immediately bonded without the application of any antioxidant; teeth in group 3 were immersed in artificial saliva at 37 °C (changed daily) for 1 week before bonding without the application of any antioxidant; teeth in group 4 were immersed in artificial saliva at 37 °C (changed daily) for 2 weeks before bonding without application of any antioxidant; teeth in group 5 were treated with 10% α-T solution immediately after bleaching before bonding; teeth in group 6 were treated with 10% GT solution immediately after bleaching before bonding; and teeth in group 7 were treated with 10% SA solution immediately after bleaching before bonding.
Bleaching procedures
The buccal surface of each tooth in all six experimental groups was exposed to 40% chemical-activated HP gel (POWER WHITENING YF; WHITEsmile, GmbH, Birkenau, Germany). According to the manufacturer’s instructions, bleaching was performed in three consecutive sessions, each for 15 min. A 1–2-mm-thick layer was applied with continuous stirring every 5 min with a pointed sterile probe countervailing the evaporated agent. The bleaching gel was then aspirated with high-power dry suction after each session, and the specimens were rinsed with water after the last session and dried.
Preparation of antioxidant solutions
To prepare 10% α-T solution, 23 mL of α-T retrieved from capsules (Spondex; Atos Pharma, Cairo, Egypt) was dissolved in 230 mL of ethanol. To prepare 10% GT solution, 23 g of GT retrieved from grinding pills (Nature’s Garden Green Tea Extract; Nature’s Garden, Holland and Barrett, Nuneaton, UK) was dissolved in 230 mL of distilled water. To prepare the 10% SA solution, 23 g of SA powder (prepared at the Chemistry Department, Faculty of Agriculture, Mansoura University, Mansoura, Egypt) was dissolved in 230 mL of distilled water. Each solution of antioxidant was kept in a sealed, dark glass sampling bottle and used fresh.
Application of antioxidants
Each antioxidant solution was applied once on the bleached buccal surface of teeth for 15 min at a continuous rate of 1 mL/minute using a syringe. Specimens treated with GT and SA were then rinsed with distilled water and those treated with α-T were rinsed with ethanol. All specimens were air-dried gently before the bonding procedures.
Bonding procedures
Etching with 37% phosphoric acid gel (N-Etch; Ivoclar Vivadent Inc., Amherst, NY, USA) was performed for 30 s on the buccal surface of the teeth. Then, the teeth were rinsed with water/air spray for 30 s and dried thoroughly until they had a frosty white appearance. Next, the primer (Transbond™ XT Light Cure; 3 M Unitek, Monrovia, CA, USA) was applied to the enamel surface using a microbrush.
Standard edgewise metallic premolar brackets with a 0.022-inch slot (American Orthodontics, Sheboygan, WI, USA) were attached to the centres of the buccal surfaces of the teeth with the adhesive system (Transbond™ XT Light Cure; 3 M Unitek, Monrovia, CA, USA). Each bracket was loaded onto the enamel surface using a bracket holder and pressed through the bracket slot perpendicularly on the bracket base by the flat end of the tensiometer (75.02.008; Morelli Ortodontia, Sorocaba, Brazil) with a standard force of 300 g. Excess adhesive was removed using the back of a bracket holder. Then, light curing (BlueLEX LD-105, Monitex Industrial Co., Taipei, Taiwan) with an intensity of 1000 mW/cm2 verified using a curing radiometer (Bluephase Meter II, Ivoclar Vivadent Inc., Amherst, NY, USA) before each application, was performed for 40 s (20 s on the occlusal surface and 20 s on the gingival surface). All specimens were stored by immersing them in artificial saliva in a sealed container at 37 °C for 24 h until polymerization was complete [21].
Thermal cycling
After 24 h of bonding, the specimens were subjected to 500 thermal cycles [21] in water baths, with the temperature of 5 °C and 55 °C and a dwell time of 30 s at each temperature and 15 s for air transfer (ROBOTA Industries, Alexandria, Egypt), representing 10–25 clinical days [22].
SBS test
A Universal testing machine (Model 3345 with Instron Bluehill 3 software; Instron Inc., High Wycombe, UK) was used for the SBS test with a compressive force, which was in the occlusal–gingival direction onto the tooth surface–bracket base interface with a crosshead speed of 1 mm/min until the bracket was detached. The fracture load was determined in newtons and divided by the surface area (10.55 mm2) of the bracket base to convert to mega-pascals (MPa).
Adhesive remnant index
To authenticate the fracture mode of the specimens, the adhesive remaining on the enamel surface was evaluated by two examiners using a stereomicroscope (SZ2-ILST; Olympus, Tokyo, Japan) under 10× magnification. The adhesive remnant index (ARI) was classified as follows [23]:
-
0: No resin remained on the enamel.
-
1: Less than 50% of resin remained on the enamel.
-
2: More than 50% of resin remained on the enamel.
-
3: All resin remained on the enamel.
Statistical analysis
One-way analysis of variance was performed to compare the SBS values of the study groups after using Shapiro–Wilk’s test to confirm the normality of the data (p > 0.050). Significant results were evaluated with post hoc Tukey’s honestly significant difference (HSD) test to determine where that difference subsisted. The Kruskal–Wallis H test was applied to compare the ARIs of the study groups after using the Kappa test (0.857) to assess inter-examiners’ reliability, which confirmed a non-significant difference. Significant results were subjected to pairwise comparisons with Bonferroni’s correction-adjusted p values. The significance level (p-value) for applied tests was ≤ 0.050. All data were processed with SPSS Statistics, version 26.0 (IBM Corporation, Armonk, NY, USA).
Results
The comparison between SBSs of the groups (means ± standard deviations), listed in Table 1, revealed a significant difference among the study groups. Pairwise comparisons of SBSs revealed that the SBSs of experimental groups 2 (immediate bonding) and 3 (1-week delay) differed significantly from that of the control group (p < 0.001), whereas the SBS of the control group did not differ significantly from those of groups 4 (2-week delay; p = 1.000), 5 (10% α-T treatment; p = 0.829), 6 (10% GT treatment; p = 0.755), and 7 (10% SA treatment; p = 0.695). Furthermore, the SBS of group 4 did not differ significantly from that of group 5 (p = 0.964), group 6 (p = 0.931), or group 7 (p = 0.899), and the SBSs of groups 5, 6, and 7 revealed no significant difference from one another (p = 1.000).
The results of stereomicroscopic analysis of the specimens are presented in Fig. 2. The frequencies of ARIs of 0 and 1 were high in groups 2 and 3, which differed significantly from those in the control group (p < 0.05). By contrast, the frequencies of ARIs of 3 and 4 were high in groups 5, 6, and 7, with no significant difference from the control group (p > 0.05).
Discussion
Although tooth bleaching has a great aesthetic value, it has some deleterious effects on tooth structures, such as increased porosity, decreased microhardness, hypersensitivity, and reduced bond strength of orthodontic brackets to tooth structure [2, 24,25,26]. Vital teeth can be bleached with HP or carbamide peroxide at different concentrations. HP in high concentrations has been reported to have more adverse effects on tooth structures than carbamide peroxide [7, 27].
In the present study, SBS reduced significantly when bonding was performed immediately after 40% HP bleaching. When bleaching was followed by a 1-week delay before bonding, SBS showed some improvement. However, a 1-week delay was not comparable enough with that in the unbleached control group, whereas a 2-week delay seemed to be appropriate for the recovery of SBS after bleaching. According to Dishman et al. [4], SBS is reduced after HP bleaching because the residual free radicals interfere with complete resin polymerization. Perdigão et al. [28] referred to the reduction in SBS as the alteration in organic/inorganic enamel structure. Electron microscopy revealed poor, sparse, and defective resin tags in bleached enamel but not in unbleached enamel [29, 30]. The reduction in SBS was considered temporary because free radicals of HP that substitute hydroxyl ions in apatite crystals eventually dissociate from those crystals [31, 32].
Different modalities have been used to neutralize the residual free radicals of bleaching and determine an alternative to delay in bonding. Results of several studies have indicated that various antioxidants can reverse the reduction in SBS after bleaching [10, 12,13,14,15, 33]. α-T represents the most potent form of vitamin E and acts as the first line of cell defense in the human body against free radicals. It is a lipid-soluble carotenoid that is easily soluble in ethanol [10, 15, 16]. The antioxidant properties of GT are attributed to flavonoids, which represent 94% of its phenolic content and include various catechins, such as catechin, epicatechins, and epigallocatechin-3-gallate. GT has been described as a promising agent with proven efficiency against the adverse effects of bleaching [16, 17]. SA is a biocompatible, non-enzymatic, neutral salt of ascorbic acid [19]. Moreover, 10% SA is considered the “gold-standard” of post-bleaching antioxidants. Lai et al. [18] reported its efficacy on dentin bonding strength after bleaching with HP or sodium hypochlorite.
The action of the α-T, GT, and SA solutions have been compared in terms of different factors, including time of application, concentrations, and physical forms to determine the most efficient protocol to be used after HP bleaching. Controversy exists in the literature about the influence of these factors in boosting SBS. Freire et al. [19] emphasized that a 5-min application of SA after HP bleaching was sufficient. In another study, Freire et al. [34] obtained the same result through two 1-min applications of 35% SA after HP bleaching. In some previous studies, a 10-min antioxidant application was considered sufficient for restoring SBS after bleaching [35, 36], whereas in others, a 10-min application of antioxidants after HP bleaching improved SBS but did not help it recover in comparison with the unbleached teeth [10, 37]. Other studies have shown that a 15-min application of antioxidants at a concentration of 10% after HP bleaching restored bonding strength [9, 38, 39]. Antioxidant application for at least 60 min was reported to counteract bleaching effects [40]; however, 60 min is considered too long for clinical application. Another study revealed that prolonged SA application reduced bond strength because acid etching could not eliminate the precipitated remnants of SA crystals [41]. Lai et al. [42] recommended that one-third of bleaching time would be convenient for antioxidant application to reverse the reduction in SBS. Therefore, in this study, a 15-min application of antioxidants seemed adequate after three 15-min bleaching sessions.
Türkün et al. [43] reported that 2.5% and 5% of SA were not sufficient to improve SBS, whereas 10% SA was sufficient. Briso et al. [44] found that 10% SA was effective after carbamide peroxide bleaching but not after HP bleaching, whereas Vidhya et al. [36] revealed that 10% SA was sufficient after HP bleaching. Solution and gel forms of antioxidants were reported to eventually reverse the bleaching effect [45]. However, Sasaki et al. [15] reported that the solution form of α-T was more effective for SBS after bleaching than the gel form. The gel form may be easier to apply clinically but could slow down the rate of antioxidant release and thus prolong the application of antioxidants [43].
Therefore, this study analyzed the antioxidants in standardized 10% concentrations for 15 min in a solution form as the target was to obtain an efficient protocol with both the lowest concentration and the minimum application time to avoid overexposure. The findings of this study revealed that 15-min applications of 10% α-T, 10% GT, and 10% SA restored bond strength after 40% HP bleaching and that the effects of a 2-week delay in bonding did not differ significantly from those of the unbleached control group. These findings were similar to those of Murad et al. [38], although they used SA in the gel form after 37.5% HP bleaching. Thus, further investigations should be conducted for determining the correlation between the time of application of both HP bleaching and antioxidants, regardless of the concentration of HP bleaching or the physical form of the antioxidants.
This study did not reveal any significant difference in SBS among the three antioxidant-treated groups. However, SBS was non-significantly higher with α-T, which may be attributed to the synergistic effect of ethanol used for its dissolution, in concordance with the finding of Sasaki et al. [15], and was non-significantly higher with GT than with SA. Nari-Ratih and Widyastuti [10] concluded that α-T and SA application resulted in non-significantly higher SBS than did GT application and stated that the difference may be attributed to the molecular weights of these agents. Antioxidants were applied for different times in the two studies, which could account for the difference in findings and suggest that longer application of GT is required to achieve its effect. Therefore, further studies are required to explain the non-significant differences among antioxidant-treated groups.
The adequate bond strength of metallic orthodontic brackets has been reported to range from 5.9 to 7.8 MPa for in-vivo and 4.9 MPa for in-vitro performance [46]. Therefore, the recorded SBSs of all groups in this study could be adequate for metallic orthodontic brackets bonding.
To determine the fracture mode of specimens, ARI was evaluated [23] using stereo-microscopy with 10× magnification. An ARI score of 2 (more than 50% of resin remained on enamel) was predominant in experimental groups 4, 5, 6, and 7, with no significant difference in comparison with the control group. In contrast, in groups 2 and 3, ARI scores of 0 (no resin remained on enamel) and 1 (less than 50% resin remained on enamel) predominated, which could be correlated to the reduction in SBS after bleaching.
Therefore, according to these findings, at least a 2-week delay in bonding is a superior protocol for completely restoring SBS after 40% HP bleaching; the discussed protocol involving antioxidants is an effective alternative.
In this study, specimens of human teeth were used, exposed to 500 thermal cycles, and preserved in artificial saliva at 37 °C to simulate a clinical situation. However, it was an in vitro study. Thus, more clinical trials are recommended with a variety of concentrations, application times, and bleaching agents. Furthermore, different antioxidants are recommended to be compared in terms of color analysis to determine how much they alert the bleached shade. The availability of commercial products containing antioxidants with a long shelf-life and stability at room temperature is required.
Conclusions
-
Application of α-T, GT, and SA had a similar efficacy in restoring SBS after 40% HP bleaching.
-
Application of α-T, GT, or SA in 10% concentration for 15 min immediately after bleaching provided an alternative to delay in bracket bonding after bleaching.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- HP:
-
Hydrogen peroxide
- SBS:
-
Shear bond strength
- α-T:
-
Alpha-tocopherol
- GT:
-
Green tea extract
- SA:
-
Sodium ascorbate
- ARI:
-
Adhesive remnant index
- Tukey’s HSD:
-
Tukey’s honestly significant difference.
References
Harlan AW. The removal of stains from the teeth caused by the administration of medicinal agents and the bleaching of pulpless teeth.J Am Med Assoc. 1884;4:123–5.
Carey CM. Tooth whitening: what we now know.J Evid Based Dent Pract. 2014;14 suppl 1:70 – 6.
Ozdemir ZM, Surmelioglu D. Bleaching agents as toxic compounds and biomarkers of damage. In: Patel VB, Preedy VR, Rajendram R, editors. Biomarkers in toxicology, biomarkers in disease: methods, discoveries and applications. Cham: Springer; 2022. pp. 1–24.
Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994;10:33–6.
de Almeida A, Lima D, Pereira A, Sousa S, Alves C. Influence of delay between dental bleaching with 35% hydrogen peroxide and orthodontic brackets on the bond strength at the enamel/adhesive interface. J Clin Exp Dent. 2019;11:e447–51.
Nascimento GCR, de Miranda CA, Machado SMM, Brandão GAM, de Almeida HA, Silva CM. Does the time interval after bleaching influence the adhesion of orthodontic brackets? Korean J Orthod. 2013;43:242–7.
Pathak K, Kumar P, Choudhary A, Shekh TM, Gosai P, Patnana AK. Comparative analysis of shear bond strength of composites to the sodium ascorbate hydrogel-treated bleached enamel surfaces: an in vitro analysis. Int J Clin Pediatr Dent. 2021;14:741–7.
Tiwari S, Dharmadeep G, Shetty KS, Shiri D. Effect of green tea and sodium ascorbate on the shear bond strength of orthodontic brackets bonded on bleached enamel: an in-vitro study. J Oral Biol Craniofacial Res. 2022;12:204–7.
Aristizábal JF, González APP, McNamara JA. Improving shear bond strength of metallic brackets after whitening. Dent Press J Orthod. 2020;25:38–43.
Nari-Ratih D, Widyastuti A. Effect of antioxidants on the shear bond strength of composite resin to enamel following extra-coronal bleaching. J Clin Exp Dent. 2019;11:e126–32.
Cheng Y, Musonda J, Cheng H, Attin T, Zheng M, Yu H. Effect of surface removal following bleaching on the bond strength of enamel. BMC Oral Health. 2019;19:50.
Xu Y, Zhou J, Tan J. Use of grape seed extract for improving the shear bond strength of total-etching adhesive to bleached enamel. Dent Mater J. 2018;37:325–31.
Zhang H, Shao S, Du A, Wang Y, Cheng B, Zhang Z. Comparative evaluation of two antioxidants on reversing the immediate bond strength of bleached enamel: in vitro study. Med Sci Monit. 2020;26:1–8.
Rahman H, Ansari MI, Khangwal M, Solanki R, Mansoori S. Comparative evaluation of 6% cranberry, 10% green tea, 50% aloe vera and 10% sodium ascorbate on reversing the immediate bond strength of bleached enamel: in vitro study. J Oral Biol Craniofacial Res. 2021;11:107–12.
Sasaki RT, Flório FM, Basting RT. Effect of 10% sodium ascorbate and 10% alpha-tocopherol in different formulations on the shear bond strength of enamel and dentin submitted to a home-use bleaching treatment. Oper Dent. 2009;34:746–52.
Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–47.
Messias DC, da Silva MFB, de Faria NS, Dias-Arnez TR, Rached-Júnior FJ, Sousa ABS. Effect of epigallocatechin-3-gallate and thermal cycling on the bond strength of resin cements to the root dentin. Odontology. 2021;109:854–9.
Lai SCN, Mak YF, Cheung GSP, Osorio R, Toledano M, Carvalho RM, et al. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001;80:1919–24.
Freire A, Souza EM, de Menezes Caldas DB, Rosa EAR, Bordin CFW, de Carvalho RM, et al. Reaction kinetics of sodium ascorbate and dental bleaching gel. J Dent. 2009;37:932–6.
Dionysopoulos D, Strakas D, Koliniotou-Koumpia E, Koumpia E. Effect of Er,Cr:YSGG laser irradiation on bovine enamel surface during in-office tooth bleaching ex vivo. Odontology. 2017;105:320–8.
International Organization for Standardization. ISO TR 11405. Dental materials—Guidance on testing on adhesion to tooth structure. Geneva, Switzerland: ISO; 1994.
Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent. 1999;27:89–99.
Årtun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod. 1984;85:333–40.
Briso ALF, Toseto RM, Rahal V, dos Santos PH, Ambrosano GMG. Effect of sodium ascorbate on tag formation in bleached enamel. J Adhes Dent. 2012;14:19–23.
Ferreira A, Batista A, Neto J, Simões T, da Silva M, de Barros D, et al. Evaluation of dental enamel microproperties after bleaching with 35% hydrogen peroxide and different light sources: an in vitro study. J Clin Exp Dent. 2021;13:e969–74.
de Oliveira Duque CC, Soares DG, Basso FG, Hebling J, de Souza Costa CA. Influence of enamel/dentin thickness on the toxic and esthetic effects of experimental in-office bleaching protocols. Clin Oral Investig. 2017;21:2509–20.
Patusco VC, Montenegro G, Lenza MA, Alves de Carvalho A. Bond strength of metallic brackets after dental bleaching. Angle Orthod. 2009;79:122–6.
Perdigão J, Francci C, Swift EJ, Ambrose WW, Lopes M. Ultra-morphological study of the interaction of dental adhesives with carbamide peroxide-bleached enamel. Am J Dent. 1998;11:291–301.
Titley KC, Torneck CD, Smith DC, Chernecky R, Adibfar A. Scanning electron microscopy observations on the penetration and structure of resin tags in bleached and unbleached bovine enamel. J Endod. 1991;17:72–5.
Sundfeld RH, Briso ALF, De Sá PM, Sundfeld MLMM, Bedran-Russo AKB. Effect of time interval between bleaching and bonding on tag formation. Bull Tokyo Dent Coll. 2005;46:1–6.
Srinivasan S, Kadandale S, Vishwanath S, Murugesan K, Parthasarathy R, Thanikachalam Y. Comparative evaluation of outcome of natural antioxidants on shear bond strength of composite bonded to bleached enamel: an in vitro study. J Pharm Bioallied Sci. 2022;14:638–43.
Zhao H, Li X, Wang J, Qu S, Weng J, Zhang X. Characterization of peroxide ions in hydroxyapatite lattice. J Biomed Mater Res. 2000;52:157–63.
Rodríguez-Barragué J, Vola-Gelmini J, Skuras-Siedemburg M, Rivera-Gonzaga JA, Cuevas-Suarez CE. Natural antioxidants to restore immediate bond strength to bleached enamel: systematic review and meta-analysis of in vitro studies. J Esthet Restor Dent. 2021;33:702–12.
Freire A, Durski MT, Ingberman M, Nakao LS, Souza EM, Vieira S. Assessing the use of 35% sodium ascorbate for removal of residual hydrogen peroxide after in-office tooth bleaching. J Am Dent Assoc. 2011;142:836–41.
Bulut H, Turkun M, Kaya AD. Effect of an antioxidizing agent on the shear bond strength of brackets bonded to bleached human enamel. Am J Orthod Dentofac Orthop. 2006;129:266–72.
Vidhya S, Srinivasulu S, Sujatha M, Mahalaxmi S. Effect of grape seed extract on the bond strength of bleached enamel. Oper Dent. 2011;36:433–8.
Gogia H, Taneja S, Kumar M, Soi S. Effect of different antioxidants on reversing compromised resin bond strength after enamel bleaching: an in vitro study. J Conserv Dent. 2018;21:100–4.
Murad CG, de Andrade SN, Disconzi LR, Munchow EA, Piva E, Pascotto RC, et al. Influence of 10% sodium ascorbate gel application time on composite bond strength to bleached enamel. Acta Biomater Odontol Scand. 2016;2:49–54.
Svizero N, Romani R, Soares LAC, Moraes IBL, Agulhari JE, Hipólito MAS, Di V, et al. Effects of neutralizing or antioxidant agents on the consequences induced by enamel bleaching agents in immediate resin composite restorations. J Adhes Sci Technol. 2017;31:965–76.
Kaya AD, Türkün M, Arici M. Reversal of compromised bonding in bleached enamel using antioxidant gel. Oper Dent. 2008;33:441–7.
Jung KH, Seon EM, Choi AN, Kwon YH, Son SA, Park JK. Time of application of sodium ascorbate on bonding to bleached dentin. Scanning. 2017;2017:6074253.
Lai SCN, Tay FR, Cheung GSP, Mak YF, Carvalho RM, Wei SHY, et al. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002;81:477–81.
Türkün M, Celik EU, Kaya AD, Arici M. Can the hydrogel form of sodium ascorbate be used to reverse compromised bond strength after bleaching? J Adhes Dent. 2009;11:35–40.
Briso ALF, Rahal V, Sundfeld RH, dos Santos PH, Alexandre RS. Effect of sodium ascorbate on dentin bonding after two bleaching techniques. Oper Dent. 2014;39:195–203.
Kimyai S, Valizadeh H. The effect of hydrogel and solution of sodium ascorbate on bond strength in bleached enamel. Oper Dent. 2006;31:496–9.
Reynolds IR. A review of Direct Orthodontic Bonding. Br J Orthod. 1975;2:171–8.
Acknowledgements
The authors would like to thank both the Analytical Chemistry Department, Faculty of Pharmacy, and the Chemistry Department, Faculty of Agriculture, Mansoura University, Mansoura, Egypt, for their assistance in preparing the artificial saliva and antioxidant solutions, respectively. The authors also thank The Egyptian Knowledge Bank (EKB) for language editing via Enago™ English Editing Service.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
SSZ performed the practical work, collected, and analyzed the data, and wrote the manuscript. SMG and MSS supervised the practical work and reviewed the manuscript. MAT reviewed the manuscript. All authors contributed to the study design. All authors have read and approved the manuscript in its final form.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study, were carried out in accordance with relevant ethical guidelines and regulations of Helsinki Declaration. Institutional approval for this study was acquired from the ethical committee of Mansoura University Dental Faculty, Mansoura, Egypt (#A08030821). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zaki, S.S., Ghorab, S.M., Tawfik, M.A. et al. Can antioxidant treatment replace delay in bracket bonding? An in vitro study. BMC Oral Health 23, 197 (2023). https://doi.org/10.1186/s12903-023-02894-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-023-02894-3