Abstract
Background
The efficacy of mouth-rinses strongly depends upon their substantivity. The use of natural and non-toxic products that avoid secondary effects is gaining interest in preventive dentistry. The purpose of this study was to evaluate the substantivity of two formulations of mouth-washing solutions based on cetylpyridinium (CPC) and O-cymen-5-ol.
Methods
This was a randomized, double-blind, crossover trial conducted at the Faculty of Medicine and Health Sciences of the University of Barcelona. Bacterial re-colonization was followed by live/dead (SYTOTM9 + propidium iodide) bacterial staining and measured by confocal laser scanning microscopy and fluorometry. Unstimulated saliva samples were collected from 16 healthy individuals at baseline saliva and then, at 15 min, 30 min and 1, 2, 3, and 4 h after the following mouth-rinses: (i) a single, 1-min mouth-rinse with 15 ml of placebo (negative control); (ii) a single, 1-min mouth-rinse with 15 ml of CPC (0.05%) ; (iii) a single, 1-min mouth-rinse with 15 ml of O-cymen-5-ol (0.09%); (iv) a single, 1-min mouth-rinse with 15 ml of CPC (0.05%) + O-cymen-5-ol (0.09%).
Results
Proportion of dead bacteria was significantly higher for all mouthrinses during the first 15 min compared to baseline (CPC = 48.0 ± 13.9; 95% CI 40.98–56.99; p < 0.001, O-cymen-5-ol = 79.8 ± 21.0; 95% CI 67.71–91.90; p < 0.05, CPC + O-cymen-5-ol = 49.4 ± 14; 95% CI 40.98–56.99; p < 0.001 by fluorometry and 54.8 ± 23.0; 95% CI 41.50–68.06; p < 0.001, 76.3 ± 17.1; 95% CI 66.36–86.14; p < 0.001, 47.4 ± 11.9; 95% CI 40.49–54.30; p < 0.001 by confocal laser scanning microscopy, respectively). Nevertheless, after 4 h, CPC + O-cymen-5-ol was the only one that obtained significant values as measured by the two quantification methods used (80.3 ± 22.8; 95% CI 67.15–93.50; p < 0.05 and 81.4 ± 13.8; 95% CI 73.45–89.43; p < 0.05). The combined use of CPC + O-cymen-5-ol increased the substantivity of the mouthrinse with respect to mouthrinses prepared with either of the two active products alone.
Conclusion
The synergistic interaction of CPC and O-cymen-5-ol prolongs their substantivity. The resulting formulation may be as effective as other antimicrobials, such as triclosan or chlorhexidine, but without their undesirable secondary effects. Thus, mouthrinsing products based on Combinations of CPC and O-cymen-5-ol may replace in the near future Triclosan and Chlorhexidine—based mouthrinses.
Similar content being viewed by others

Introduction
Oral biofilms are multi-species communities of microorganisms existing as complex ecosystems on both hard and soft tissues of the oral cavity [1,2,3]. Both the effective removal of biofilms and the prevention of their formation are essential to maintain good oral health [4]. One of the main strategies is to control the formation of young biofilms, as they are much easier to eradicate than mature ones.
Mechanical approaches, such as brushing and flossing, are essential to control dental biofilms [5]. Nevertheless, patients’ efforts to maintain good biofilm control are often hampered by the difficulty of accessing interdental areas and gingival margins [6]. Antimicrobial mouth-rinses complement mechanical oral hygiene regimens by improving biofilm removal [7,8,9].
Cetylpyridinium chloride (CPC) is a cationic quaternary ammonium compound that has been proposed as an alternative to triclosan and chlorhexidine (CHX), given the latter’s adverse effects, such as tooth staining, taste disturbance, mouth ulcers, burning and even presumptive carcinogenicity [10, 11]. CPC is effective in controlling dental plaque and in preventing gingivitis, based on its significant bactericidal effect on the microorganisms involved in periodontal diseases. While CPC is less bactericidal than CHX, its fewer side effects, mostly related to aesthetics, are the main reason for the increasing interest in its use. The cationic nature of CPC accounts for its antibacterial effect, as it allows binding to negatively charged bacterial surfaces and to the negatively charged proteins of oral tissues [12, 13].
Crude essential oils derived from plants (thyme, oregano, cinnamon, and others) have been classified as GRAS (generally recognized as safe) by the FDA (United States Food and Drug Administration) and accepted as usable in food by the European Union. The use of phytocompounds, including essential oils, as oral disinfectants (or antimicrobials) is gaining interest due to their demonstrated antimicrobial activity and the value currently placed by consumers on natural products [14]. For example, essential oils have been examined for their antibacterial activity in the treatment of periodontitis, including that caused by Aggregatibacter actinomycetemcomitans [15]. O-cymen-5-ol (CH3)2CHC6H3(CH3)OH) is a natural phenolic compound derived from isopropyl cresol and its properties suggest its potential as an agent to maintain buccal health [16,17,18].
An important quality of mouth rinses is substantivity, defined as the persistence of antimicrobial action in the mouth over time. Substantivity requires adsorption of the agent on buccal surfaces, which implies the late control of biofilm regrowth mostly via bacteriostatic activity [19]. Among the multiple factors that determine substantivity is the control or alteration of adhesion, the duration of the preparation’s antimicrobial activity, and the contributions of synergisms, antagonisms, etc. To improve substantivity and therefore the antimicrobial effectiveness, the addition of different essential oils against microorganisms involved in oral diseases has recently been proposed [20]. Thus, in this work we quantitatively compared the substantivity of oral rinses consisting of: (i) O-cymen-5-ol + CPC, (ii) CPC, and (iii) O-cymen-5-ol in the elimination of salivary microbiota up to 4 h after their use. To our knowledge no previous studies on substantivity of CPC and O-cymen-5-ol have been published.
Materials and methods
This was a randomized, double-blind, crossover study comparing the in situ persistent antibacterial effect (substantivity) of O-cymen-5-ol + CPC, O-cymen-5-ol, and CPC on the salivary microbiota.
Selection of the study group
The study group comprised 16 adult volunteers between 20 and 45 years of age. All participants had a good oral health status, including a minimum of 24 evaluable permanent teeth with an intact periodontium [pockets ≤ 3 mm and the absence of gingival hemorrhage, according to the criteria of Dietrich et al. [21] and the absence of caries. The exclusion criteria were: smoking, any type of dental prosthesis or orthodontic device, Sjögren syndrome, allergies to oral hygiene products, antibiotic treatment or the routine use of oral antiseptics during the previous 3 months, and the presence of any systemic disease that could lead to an alteration in the production and/or composition of the saliva. All volunteers followed the protocol shown in Fig. 1. Assignment to groups was performed randomly using an Internet-based balanced randomization system (Dallal GE. Available from: www.randomization.com). This study was approved by the Ethics Committee of the Hospital Odontològic Universitat de Barcelona (CEIm, no. 22/2021) and registered at ClinicalTrials.gov PRS, NCT05365737 (09/05/2022).
Mouthrinse treatment
The volunteers used each of the four mouth-rinses as described below with a one-week resting period between rinses. Written informed consent was obtained from all of the volunteers. Each of the protocols was carried out between May and June 2022 at the Pavellò de Govern (Faculty of Medicine and Health Sciences) of the University of Barcelona.
The volunteers refrained from any type of oral hygiene beginning at midnight the day before the experiment. On the day of the experiment, they were not allowed to eat or drink before or during the experiment. One of the researchers supervised the participants in a living room during the experimental period.
The spitting method [22] was used to collect unstimulated saliva (2 ml) from each volunteer at baseline (BS) and then 15 min, 30 min and 1, 2, 3, and 4 h after the supervised use of the following mouth-rinses: (i) a single, 1-min mouth-rinse with 15 ml of placebo (negative control); (ii) a single, 1-min mouth-rinse with 15 ml of CPC (0.05%); (iii) a single, 1-min mouth-rinse with 15 ml of O-cymen-5-ol (0.09%); and (iv) a single, 1-min mouth-rinse with 15 ml of CPC (0.05%) + O-cymen-5-ol (0.09%).
Processing of saliva samples
To remove the epithelial cells from the saliva samples and fluidify the mucus, 1 ml of dithiothreitol (Sputolysin®, Merck, Darmstadt, Germany) was mixed with the saliva samples (1 ml) in a test tube. After a 1-h incubation at 37 °C, the treated samples were filtered through sterile filters (50-µm pore size; CellTrics®, Sysmex, Goerlitz, Germany). The resulting filtrates were then further analyzed.
To establish bacterial viability, the LIVE/DEADTM BacLight kit (Molecular Probes, Leiden, The Netherlands), which is composed of a cell-permeant cyanine dye (SYTOTM9) and propidium iodide (PI), was used. SYTOTM9 enters viable and non-viable cells due to its ability to penetrate intact membranes, while PI penetrates only those cells with a high membrane permeability (i.e. damaged cells). Both dyes stain nucleic acids and may be monitored on the basis of their fluorescence. SYTOTM9 and PI were mixed at a ratio of 1:1 (6 µL of SYTOTM9 and 6 µL of PI in 2 ml of filter-sterilized dH2O) and the viability of the treated cells was measured based on fluorometric analysis and confocal laser scanning microscopy (CLSM) as described below.
Fluorometry
One-hundred µL of the processed sample was pipetted into separate wells of 96-well black microtiter plates; 100 µL of the LIVE/DEADTM solution was added to the wells using a new tip for each one. Fluorescence was measured using a Fluostar Optima plate reader (BMG Labtech Ortenberg, Germany) based on emission 1 (green), determined at an excitation wavelength of 485 nm and an emission wavelength of 530 nm, and emission 2 (red), using 485-nm and 620-nm filters, respectively. The percent bacterial viability was estimated by comparison with a growth curve derived from a mixture of Escherichia coli and Staphylococcus aureus, as outlined in the LIVE / DEAD TM BacLight manual. Green/red fluorescence ratios were calculated for each proportion of live/dead bacteria. The equation to establish viability was y = x * 0.0045 + 0.3383, where x is living bacteria and y is the green/red fluorescence ratio.
CLSM
One-ml samples were centrifuged in Eppendorf tubes at 10,000 × g for 10 min. The supernatant was discarded while the pellet was resuspended in 100 µl of LIVE/DEADTM solution and stored in the dark at room temperature for 15 min. Ten µl of the stained bacterial suspension was placed on a slide and covered with a 22 × 22 mm coverslip. Bacterial cells were visualized using a confocal laser scanning microscope (Leica TCS-SL, Leica Microsystems, Mannheim, Germany) equipped with a 488-nm argon laser and 543-nm and 633-nm He/Ne lasers (Centres Científics i Tecnològics, Universitat de Barcelona, Barcelona, Spain). Six fields/samples were selected by the operator (FA) and examined using a 63× oil immersion objective. The image resolution was 1024 × 1024 pixels. Image processing and analysis, including quantification of live and dead bacteria, were performed using the Open Source software project Fiji (Fiji Is Just ImageJ). Cell nuclei and bacterial aggregates were excluded.
Statistical analysis
The intraclass correlation coefficient (ICC) test was used to determine intra- and inter-observer correlations for CLSM analysis. The mean and standard deviation (%) of viable bacteria were calculated. The percentages were adjusted according to the baseline, set as 100%. A repeated-measures ANOVA and simple comparisons were used between groups and time points. Differences were considered statistically significant at a p-value < 0.05. The data were statistically analyzed using Rstudio (v1.2.1335 for Mac OS).
Results
Both CLSM visualization and the subsequent quantification of viable and dead bacteria showed differences in bacterial viability over time between mouth-rinses (Fig. 2).
In the intra- and inter-observer correlation analysis of the CLSM results, the mean ICC was 0.85 (p < 0.001) and 0.80 (p < 0.001) respectively.
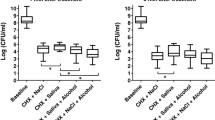
The largest decrease in salivary bacterial viability was obtained after a single application of the mouth-rinse containing CPC + O-cymen-5-ol, followed by that containing CPC alone, O-cymen-5-ol alone, and placebo (Figs. 3 and 4). The results of the analyses of bacterial viability and of the effect of the mouth-rinses on the salivary microbiota, tested at different times using fluorometry and CLSM, are shown in Tables 1 and 2, respectively.
Fluorometry revealed significant differences when compared the three mouth rinses to BS; following the use of the CPC and CPC + O-cymen-5-ol during 3 and 4 h, respectively (p < 0.05). O-cymen-5-ol alone had significant effect only during the initial 30 min. Similar results were obtained with CLSM. Nevertheless, O-cymen-5-ol decreased alive bacteria proportion significantly during the first hour (p < 0.05).
Comparisons between mouth-rinses also showed significant differences for CPC and CPC + O-cymen-5-ol compared to placebo during the first 2 and 3 h, respectively (p < 0.05). On the contrary, O-cymen-5-ol did not significantly differ from placebo (p > 0.05) irrespective of the time point and measurement method.
Discussion
Essential oils have interesting antimicrobial and other bioactivities including, antiviral, antioxidant, and anticancer activities [23]. Their main use thus far has been in food technologies, with recent expansions into pharmaceuticals and cosmeceuticals, reflecting the rapidly growing demand by consumers for “natural products.” There has also been a move to replace CHX and triclosan with CPC, especially after CPC was shown to inactivate the COVID-19 coronavirus in the oral cavity [24]. Accordingly, both O-cymen-5-ol and CPC have garnered interest as replacements for traditional mouth-rinses with antibacterial activity.
Substantivity is a critical property of mouth-rinses. Typically, the efficacy of antiseptics is evaluated in in vitro suspensions or in other specifically designed tests or in tests similar to those used to assess antibiotics. However, the substantivity (persistence) of an antimicrobial cannot be quantified in vitro but must necessarily be investigated in vivo. In this study, we evaluated and compared the substantivity of two putative components of the new generation of mouth-rinses, alone and in combination.
The substantivity of CPC-containing mouth-rinses on the salivary microbiota has been examined in a few reports. Elworthy et al. [19] found that the tested CPC rinses were similar and all were significantly more substantial than the control rinses between 180 and 300 min. The same authors found that additional components, such as fluoride and/or alcohol, did not substantially enhance the activity of CPC. In spite we failed in demonstrating synergistic effect of CPC and O-cymen 5 ol in terms of Minimal inhibitory concentrations (data not shown), we succeed in demonstrating that the combination of active molecules did maintain bacterial counts at a lower level and for a longer period of time than BS.
Similar results were obtained by Roberts & Addy [25], who compared the antibacterial properties of three antiseptic mouth-rinses, containing CHX, CPC, and hexetidine, and of an alexidine preparation. A return to pre-rinse levels after 90 min was obtained with hexetidine, after 3 h with CPC, after 5 h with alexidine, and after 7 h with CHX gluconate. Residual salivary antibacterial activity remained for 90 min with CPC, for 3 h with hexetidine and alexidine, and for 5 h for CHX gluconate.
Epifluorescence microscopy has been used to assess the effect of CHX on the salivary microbiota. Quintas et al. [26] evaluated the immediate effect of a 0.2% CHX mouthwash and found a significant reduction in bacterial viability compared to BS for up to 7 h (87.6 ± 6.5% vs. 73.6 ± 8.8%; p < 0.001). Tomás et al. [27] reported significantly lower counts of viable salivary bacteria 30 s after a 0.2% CHX rinse than after the control sterile water treatment (p < 0.001), with a significant antibacterial effect achieved for up to 7 h with the former (p < 0.001). Our investigation showed significant (p < 0.05) reductions in salivary bacteria for up to 4 h following mouth-rinse treatments with CPC and O-cymen-5-ol, as demonstrated by fluorometry and CLSM.
CHX has long been the gold standard in dental mouth-rinses, based on its bactericidal properties against gram-negative and gram-positive species, yeast, and other microorganisms. However, CHX use is associated with a high incidence of adverse effects, such as tooth enamel staining, taste disturbance, and the appearance of ulcers in the mouth and tongue [12]. In addition, there is evidence of the potential resistance of oral bacteria to CHX when used as a standard antiseptic [28]. Consequently, alternative mouth-rinse formulations, such as those based on CPC and complemented by the addition of natural products, such as O-cymen-5-ol, with demonstrated substantivity should be explored.
In this research, the LIVE ⁄ DEAD TM assay was used to quantify bacterial viability. Its advantages include speed, simplicity, and the simultaneous quantification of viable and non-viable bacteria. However, the assay may determine higher percentages of nonviable bacteria than in the actual sample [29]. However, Tawakoli et al. [30] demonstrated the validity of the LIVE/DEADTM assay in estimating bacterial viability and that the combination of SYTOTM9 / PI is a reliable tool in the evaluation of multi-species biofilms. Our results showed that the LIVE/DEADTM assay can be used to analyze the effects of antimicrobials that alter the integrity of the cytoplasmic membrane in different oral ecosystems.
Among the limitations of our study was the quantification of bacterial viability by CLSM, as errors in the counts may have arisen due to bacterial aggregation, the staining of cellular elements, or the presence of contaminating material. Furthermore, microscopic examination is tedious since a large number of fields and samples must be assessed. Nevertheless, the results have been confirmed by fluorometric automatic reading emphasizing the validity of the general conclusion. In addition, in future research the substantivity of other CPC additives, tested at different concentrations, should be measured.
The main limitations of this work reside in the nature of the samples and in the possible heterogeneity of the oral bacterial populations of the different participants. The saliva carries bacteria that originate from planktonic and sessile states, so the data must be interpreted as a combined effect of the washers on both populations. In any case, the collection of other types of samples, such as biofilm scraping, also entail numerous biases due to the collection methods. On the other hand, the only data handled are those of bacteria with intact membranes (and therefore presumably live bacteria) and those that have their membranes damaged and are therefore dead bacteria. In any case, the approximation seems adequate and has already been used on other studies. The maximum times of four hours were taken based on the data obtained in pilot tests carried out prior to the beginning of the experiment. Also we decided to leave a week of rest between tests to be able to use the same volunteers and after verifying that after a week, the oral microbiota values were apparently fully restored.
In summary, our study showed a higher substantivity of the mouth-rinse CPC + O-cymen-5-ol or CPC alone than of O-cymen-5-ol alone (or placebo). The addition of O-cymen-5-ol extended the substantivity of CPC by more than 1 h (from 3 to 4 h) and significantly reduced bacterial recovery compared to baseline. Compared to placebo, substantivity was increased by the addition of O-cymen-5-ol to CPC, from 2 h (CPC) to 3 h (CPC + O-cymen-5-ol). These observations demonstrate the synergy of CPC and O-cymen-5-ol in increasing substantivity.
Availability data and materials
Data will be made available on a case-by-case basis and also additional information will be provided by contacting the Corresponding Author.
References
Marsh PD, Bradshaw DJ. Dental plaque as a biofilm. J Ind Microbiol. 1995;15:169–75.
Foster JS, Kolenbrander PE. Development of a multispecies oral bacterial community in a saliva-conditioned flow cell. Appl Environ Microbiol. 2004;70:4340–8.
Darrene L-N, Cecile B. Experimental models of oral biofilms developed on inert substrates: a review of the literature. BioMed Res Int. 2016;2016:1–8.
Aimetti M. Nonsurgical periodontal treatment. Int J Esthet Dent. 2014;9:251–67.
Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets: dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55.
Santos A. Evidence-based control of plaque and gingivitis: plaque and gingivitis control. J Clin Periodontol. 2003;30:13–6.
Brading MG, Marsh PD. The oral environment: the challenge for antimicrobials in oral care products. Int Dent J. 2003;53:353–62.
Marsh PD. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38:11–5.
Marsh PD. Contemporary perspective on plaque control. Br Dent J. 2012;212:601–6.
Sanidad KZ, Wang G, Panigrahy A, Zhang G. Triclosan and triclocarban as potential risk factors of colitis and colon cancer: roles of gut microbiota involved. Sci Total Environ. 2022;842:156776.
Tartaglia GM, Tadakamadla SK, Connelly ST, Sforza C, Martín C. Adverse events associated with home use of mouthrinses: a systematic review. Ther Adv Drug Saf. 2019;10:204209861985488.
McCoy LC, Wehler CJ, Rich SE, Garcia RI, Miller DR, Jones JA. Adverse events associated with chlorhexidine use results from the Department of Veterans Affairs Dental Diabetes Study. J Am Dent Assoc. 2008;139:178–83.
Lee J-E, Lee J-M, Lee Y, Park J-W, Suh J-Y, Um H-S, et al. The antiplaque and bleeding control effects of a cetylpyridinium chloride and tranexamic acid mouth rinse in patients with gingivitis. J Periodontal Implant Sci. 2017;47:134–42.
Miranda-Cadena K, Marcos-Arias C, Mateo E, Aguirre-Urizar JM, Quindós G, Eraso E. In vitro activities of carvacrol, cinnamaldehyde and thymol against Candida biofilms. Biomed Pharmacother Biomed Pharmacother. 2021;143:112218.
Akkaoui S, Johansson A, Yagoubi M, Haubek D, El Hamidi A, Rida S, et al. Chemical composition, antimicrobial activity, in vitro cytotoxicity and leukotoxin neutralization of essential oil from Origanum vulgare against aggregatibacter actinomycetemcomitans. Pathog Basel Switz. 2020;9:E192.
Newby CS, Rowland JL, Lynch RJM, Bradshaw DJ, Whitworth D, Bosma ML. Benefits of a silica-based fluoride toothpaste containing o-cymen-5-ol, zinc chloride and sodium fluoride. Int Dent J. 2011;61:74–80.
Kakar A, Newby EE, Kakar K, Ghosh S, Targett D, Bosma ML. A randomised clinical trial to assess maintenance of gingival health by a novel dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride. Int Dent J. 2011;61:13–20.
Kakar A, Newby EE, Ghosh S, Butler A, Bosma ML. A randomised clinical trial to assess maintenance of gingival health by a novel gel to foam dentifrice containing 0.1%w/w o-cymen-5-ol, 0.6%w/w zinc chloride. Int Dent J. 2011;61:21–7.
Elworthy A, Greenman J, Doherty FM, Newcombe RG, Addy M. The substantivity of a number of oral hygiene products determined by the duration of effects on salivary bacteria. J Periodontol. 1996;67:572–6.
Tardugno R, Pellati F, Iseppi R, Bondi M, Bruzzesi G, Benvenuti S. Phytochemical composition and in vitro screening of the antimicrobial activity of essential oils on oral pathogenic bacteria. Nat Prod Res. 2018;32:544–51.
Dietrich T, Ower P, Tank M, West NX, Walter C, Needleman I, et al. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions – implementation in clinical practice. Br Dent J. 2019;226:16–22.
Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982;61:1158–62.
Sharma M, Grewal K, Jandrotia R, Batish DR, Singh HP, Kohli RK. Essential oils as anticancer agents: potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed Pharmacother Biomed Pharmacother. 2022;146:112514.
Anderson ER, Patterson EI, Richards S, Pitol AK, Edwards T, Wooding D, et al. CPC-containing oral rinses inactivate SARS-CoV-2 variants and are active in the presence of human saliva. J Med Microbiol. 2022;71(2):001508.
Roberts WR, Addy M. Comparison of the in vivo and in vitro antibacterial properties of antiseptic mouthrinses containing chlorhexidine, alexidine, cetyl pyridinium chloride and hexetidine. Relevance to mode of action. J Clin Periodontol. 1981;8:295–310.
Quintas V, Prada-López I, Donos N, Suárez-Quintanilla D, Tomás I. In situ neutralisation of the antibacterial effect of 0.2% chlorhexidine on salivary microbiota: quantification of substantivity. Arch Oral Biol. 2015;60:1109–16.
Tomás I, García-Caballero L, Cousido M, Limeres J, Álvarez M, Diz P. Evaluation of chlorhexidine substantivity on salivary flora by epifluorescence microscopy. Oral Dis. 2009;15:428–33.
Cieplik F, Jakubovics NS, Buchalla W, Maisch T, Hellwig E, Al-Ahmad A. Resistance toward chlorhexidine in oral bacteria – is there cause for concern? Front Microbiol. 2019;10:587.
Netuschil L, Auschill TM, Sculean A, Arweiler NB. Confusion over live/dead stainings for the detection of vital microorganisms in oral biofilms - which stain is suitable? BMC Oral Health. 2014;14:2.
Tawakoli PN, Al-Ahmad A, Hoth-Hannig W, Hannig M, Hannig C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin Oral Investig. 2013;17:841–50.
Acknowledgements
The authors wish to thank the Centres Científics i Tecnològics (Universitat de Barcelona, Campus de Bellvitge, Barcelona, Spain) for their technical assistance. FA thanks the Chilean Government (ANID)/Nº11040/2020. The technical support of Sonia Suarez is gratefully acknowledged.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
FA and MJ performed the sampling, staining, and CLSM; JL-L and MV conceived the research; TV and JMS designed the experiments; FHAR, MF, CT and EJ prepared the mouth-rinses; FA and MV wrote the manuscript. All authors contributed significantly to the design, conceptualization, discussion and evaluation of the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Hospital Odontològic Universitat de Barcelona Ethics Committee CEIm (N°33/2021), and registered at ClinicalTrials.gov PRS, NCT05365737. Informed consent was obtained from all volunteers who participated in this study.
Consent for publication
N/A.
Competing interests
Authors disclose all competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aguilera, FR., Viñas, M., Sierra, J.M. et al. Substantivity of mouth-rinse formulations containing cetylpyridinium chloride and O-cymen-5-ol: a randomized-crossover trial. BMC Oral Health 22, 646 (2022). https://doi.org/10.1186/s12903-022-02688-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-022-02688-z