Abstract
Background
The calcification of the tooth pulp is a pathological condition that occurs in response to various factors. A uncommon haematological condition known as paroxysmal nocturnal haemoglobinuria (PNH) is characterized by bouts of haemolysis, and it requires long-term use of glucocorticoids (GCs).
Case presentation
A female patient who was diagnosed with PNH and had a history of long-term use of GCs came to our department for root canal therapy (RCT) for teeth 25, 26, and 27. The radiographs showed generalized pulp canal obliteration (PCO) in most of the patients. None of these teeth (25, 26, or 27) were sensitive to percussion, and they did not respond to thermal or electrical sensitivity tests. A diagnose of pulp necrosis was made for these teeth. RCT was carried out with the help of an oral microscope, and then a prosthodontic procedure was created for the teeth.
Conclusions
Based on the patient’s long history use of GCs and a series of related studies, we conclude that the long-term usage of GCs contributes significantly to the onset of PCO.
Similar content being viewed by others
Introduction
Glide path is the key to infection control in RCT, but in clinical practice, root canals are often blocked due to pulp calcification, which makes accessing and cleaning the root canal system more difficult. Local and systemic factors may contribute to the formation of dental pulp calcification [1]. Excessive forces caused by trauma and clenching, the presence of restorations, cavity preparation, and caries are common local factors [2, 3]. Systemic factors include end-stage renal diseases [4], cardiovascular disease [5], and some long-term medications [1, 6, 7].
Ranjitkar et al. [8] believed that pulp calcification is an age-related disease. The aging process of tooth pulp leads to a decrease in fibroblast, odontoblast, and mesenchymal cells. Between the ages of 20 and 70, these cells have been reported to decrease by 50%. With age, secondary dentin accumulates gradually, pulp cavity and root canal become smaller, fat may deposit in the pulp, and calcification usually occurs at these deposits [9]. Therefore, age-related calcifications are mostly located in the medullary cavity, canal orifice, and upper canal segment. Some scholars have proposed a relationship between periodontal disease and pulp calcification [10], and pulp calcification is a common manifestation in the healing process after a traumatic injury. Dental-pulp calcification can be considered a kind of active repair. In the situation of tooth caries, periodontal disease, trauma and abrasion, and dental pulp produce a defensive reaction. In the defensive process, the formation of the calcification focus is centred on the degeneration and necrosis pulp cells. At the same time, the secretion of reactive dentin by odontoblasts is accelerated, eventually leading to the formation of pulp stones or diffuse calcification and blocking the root canal system [11, 12]. It has also been proposed that the blood clots formed after tooth injury will calcify and trigger calcification of the remaining pulp [13]. The location of calcification is mostly related to the origin of stimulation, so periodontal calcification usually begins at the root tip whereas stress stimulation calcification can occur throughout the root canal.
Other studies have shown that the application of calcium hydroxide and hydrogen peroxide can promote calcification [14, 15]. Some scholars isolated nanobacteria (CNPs) that can cause calcification of biological tissues in myeloid stones [16], and the presence of CNPs may promote the calcification of nuclear, thus forming a biological apatite structure or leading to cell-mineralization denaturation. Studies have also shown that dentine dysplasia can lead to dental pulp calcification, and the gene mutation of the bicistronic dentine sialophosphoprotein (DSPP) is one of the key variables contributing to dentine dysplasia [17].
In addition, long-term use of GCs has been correlated with calcification of the dental pulp, and a few related clinical reports have been reported [6, 18, 19]. According to Chigono et al. [19], a patient with systemic lupus erythematosus was taking oral GCs for a long time. They examined the patient’s premolar stumps histopathologically and found that nearly all samples had constricted dental pulp and found no odontoblasts.
A uncommon haematological disorder known as paroxysmal nocturnal haemoglobinuria (PNH) is characterized by bouts of haemolysis [20]. Because of its many forms and complex pathophysiology, it has piqued the attention of haematologists for more than a century [21, 22]. PNH is thought to affect 1–1.5 instances per million people globally, while this number may be greater in certain areas [23, 24]. The development of the illness into myelodysplastic syndromes, bone marrow failure, and thrombosis are further clinical signs of PNH [20]. Due to the clonal growth of a mutant haematopoietic stem cell, PNH is believed to originate from these non-erythroid characteristics (HSC) [25]. This fatal condition's biological anomaly results from a mutation in the phosphatidylinositol glycan class A (PIGA) gene, which causes a shortage of complement regulating proteins that are glycosylphosphatidylinositol (GPI)-anchored, such as CD55 and CD59, on blood cells' surfaces [23, 26].
The most common cause of death is thromboembolism, which is much more common in patients with PNH haemolysis [23, 27]. The traditional treatment for PNH [24] is symptomatic support therapy, and GCs are often administered to alleviate anaemia and perhaps reduce the frequency of haemolytic episodes. Other symptomatic supportive treatments include transfusions of red blood cells and platelets when necessary and antibiotics when infections occur.
Case presentation
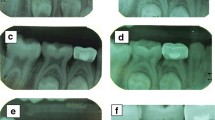
The female patient was born in 1973 and was diagnosed with PNH at the age of 21 in 1994. Since then, she has taken GCs (methylprednisolone, about 24-50 mg/day) for more than 20 years, mostly without interruption although with varying doses. The patient also took ciclosporin for a short time when she was diagnosed with PNH, and she took aspirin and calcium tablets intermittently. In 2011 and 2015, the patient had been hospitalized twice for lower-extremity venous thrombosis. In 2018, the patient experienced spontaneous and nocturnal pain in the upper left posterior teeth, and she visited a general practitioner. Although the toothache symptoms were relieved after pulp access, the general practitioner was unable to access or debride the root canals, and the patient was advised to visit our department. Teeth 25, 26, and 27 had been treated. None of these symptomatic teeth were sensitive to percussion, and testing of their thermal or electrical sensitivity revealed that they did not react. A periapical radiograph showed no obvious root canal image of the symptomatic teeth (Fig. 1a). A diagnosis of pulp necrosis was made for these teeth. The root canals of the symptomatic teeth were examined using cone-beam computed tomography (CBCT) to see whether they were entirely or partly obstructed. The CBCT images revealed that the symptomatic teeth were completely blocked and generalized PCO of the patient’s teeth had occurred (Fig. 1b, c). The patient was in a normal occlusion relationship, with no sign of wear on any of the teeth’s occlusal surfaces (Fig. 2). The patient had no history of trauma or orthodontic or surgical interventions in her teeth or jaws. The symptomatic teeth’s root canals were so obstructed that they could not be accessed or debrided. Then the patient was transferred to another endodontics expert. However, even with the help of an oral surgery microscope and oral ultrasonic equipment, only tooth 26’s mesial buccal and distal buccal canals were accessed and debrided (Fig. 3). Calcium hydroxide was applied to the symptomatic teeth for two weeks, and the AH-plus sealer and gutta-percha were used to obturate the debrided canals, and the unlocated canals went without any further treatment. Then teeth 25 and 27 were filled with composite resin and a crown was made for 25. As for tooth 26, a post–core crown was made. The patient attended a followed-up visit two years later and showed no discomfort or periapical inflammation of the treated teeth (Fig. 4). In 2021, the patient died unexpectedly at the age of 48. The probable cause of death was a pulmonary embolism due to a microthrombus, according to her haematology doctor. Figure 5 shows the entire course of the patient’s diseases.
Discussion and conclusions
GCs are widely used to treat arthritis, chronic asthma, allergies, and autoimmune diseases and following organ transplants [28]. However, long-term use of GCs may cause an increased risk of infection, osteoporosis, retardation of growth in children, and disturbances in wound healing [28, 29]. The incidence of GCs’ adverse reactions depends on the dose and, to a greater degree, the duration of treatment [28]. One possible side effect of GC therapy in the dental field is extensive narrowing of dental pulp [19].
In 1966, dental-pulp calcification was seen in the adult cortisone-pretreated rats' incisors, according to Anneroth et al. [30]. The odontoblasts' differentiation was reduced and there was a clear disarray in the cortisone-treated rats. Rats receiving treatment had more undifferentiated odontoblasts than untreated rats. The pulp tissue looked to be exceedingly cell-rich overall in the cortisone-treated rats, and the number and breadth of blood vessels increased. In certain areas of the pulp, the researchers saw an excessive growth of a hard tissue that resembled bone and was full of cellular and vascular inclusions. The pre-dentinal zone, which was substantially expanded and had an uneven border facing the dentin, also included comparable inclusions, which they also noted. In another animal experiment, Näsström et al. [4] observed that in the molars of adult GC-pretreated rats, new dentin was forming, but there was no dentin development observable in the control group. However, the results of animal experiments were not unanimous concerning GCs’ effect on dentin formation. Ball et al. [31,32,33] observed in three trials that rats given subcutaneous injections of corticosteroids had narrower dentin walls, resulting in larger pulp chambers in the incisors. After administering corticosteroids to freshly weaned rats, Johannessen et al. [34] discovered that dentin development in the molars was suppressed. However, primary dentin development seemed to be finished somewhere between the 12th and 35th day of life [35,36,37]. There seems to be a lot of diversity in the daily dentin apposition in the molars of newborn rats. Secondary dentin production begins on day 35 of the rat's life and is seen in the crown pulp under the cusps [36, 38] when secondary dentin is stimulated by attrition. Comparing the age of experimental rats in the two studies [4, 34], the difference in results may occur because Young rats (21 days) with primary dentin development underway were employed by Johannessen [34], while mature rats were used by Näsström (3.5–5 months). Moreover, it is possible that the disparate effects seen in investigations on the impact of corticosteroids on rat incisors and molars [31,32,33,34] and molars [4, 34] were brought on by the use of various corticosteroid dosages. As opposed to this, according to the results of the energy-dispersive X-ray microanalysis [4], the dentin caused by GCs was found to have the same calcium and phosphorus composition as normal dentin in the rat molars of the control group. This finding may indicate that treatment with GCs did not affect the mineralization process, and as a result, the quality of the GC-induced dentin may be equal to that of normal dentin in calcium and phosphor. A scanning microscopy study also revealed that the morphology of the GC-induced dentin in rat incisors was similar to that of naturally occurring dentin [4]. Näsström et al. [4] inferred that GCs might exceed protein synthesis and would therefore induce accelerated production of the matrix in the predentin zone. The mineralization rate could be normal, as seen in the experimental studies on rat incisors, but as the corticosteroids received continuously activate the odontoblasts, the mineralization process will proceed even in mature, formerly resting odontoblasts, as was noted in the experimental study on rat molars.
In normal adults, as the tooth ages, the coronal and root regions of the pulp chamber gradually continue to experience dentin mineralization [39]. In 51 individuals with renal disorders, the radiographic narrowing of the tooth pulp chamber was examined [40]. The findings showed that there appeared to be a relationship between dental pulp chamber narrowing, the quantity of corticosteroids received, and the pharmacokinetics (total plasma prednisolone clearance) of these drugs. This was done since there was no way to link a constriction of the tooth pulp chamber to a particular renal illness. On the other hand, an individual response to the corticosteroid treatment probably occurs [4, 40]. In a few patients, a relatively low total dose of corticosteroids started a productive reaction in the odontoblasts and caused narrowing of the dental pulp chamber whereas in others, a high total dose caused no dentin formation. The explanation for the different reactions might be the renal failure and its influence on hormone metabolism and therefore on dentin formation, which is a complex series of events not completely investigated.
Shinozuka et al. [41] compared the narrowing of dental-pulp cavity between patients under long-term steroid treatment (the steroid group) and patients who were not receiving steroids (the non-steroid group). The results indicated that the long-term administration of steroids was the main reason for the greater narrowing of dental pulp cavity in the steroid group. In our case, the patient had taken GCs for more than 20 years, and we think that the long-term administration of GCs was likely a major factor causing generalized PCO.
On the other hand, as described in this case, teeth with PCO come into the high-difficulty category of the American Association of Endodontists Case Assessment criteria if RCT is necessary [42]. In clinical practice, PCO is usually caused by dental trauma, and it usually affects young adults’ anterior teeth [43, 44]. A serious complication in teeth with PCO is pulp necrosis. Following an observational period of between 3.4 and 16 years on average, the incidence of the problem in permanent teeth with PCO varied from 1 to 16% [45,46,47,48]. The clinical crown of these teeth may also get discolored, becoming darker than the surrounding teeth in certain cases. The increasing dentine thickness, which causes the crown's translucency to diminish, is the cause of this cosmetic issue [46, 49].
Vinagre et al. [48] developed an improved clinical decision-making methodology for the treatment of PCO teeth based on the findings of a systematic review and the most current literature. This clinical decision-making algorithm demonstrated that therapy recommendations are based on clinical and radiographic symptoms and indicators, including discolouration signals.
The most common clinical strategy used for PCO teeth was watchful waiting. Additionally, the prophylactic RCT technique should not be employed as a preventative measure or as a first line of treatment for discolored, asymptomatic PCO teeth, according to the research [48]. In these situations, external bleaching needs to be the first approach used to remedy cosmetic issues. When there were indicators of periapical disease on radiographs or in symptoms, an endodontic approach was advised. Send them to an endodontist if required. Clinicians may choose for guided access or, if it's feasible, a traditional approach depending on the circumstances [50, 51]. Based on the results, endodontic microsurgery [52] or even an intentional re-implant when the surgery is not feasible [53, 54] is advised in the event of failure. The final resort is tooth extraction, which must be followed by an appropriate rehabilitation therapy.
Conclusion
Although the effects of PNH and other medications cannot be ruled out, based on the patient’s long history of GC use and a series of related studies, we conclude that the long-term usage of GCs contributes significantly to the onset of PCO. In clinical work on patients receiving GCs special care, a clinical decision-making algorithm must be used in therapy planning.
Availability of data and material
The corresponding author may provide the datasets used and/or analyzed during the present investigation upon reasonable request.
Abbreviations
- GCs:
-
Glucocorticoids.
- CNPs:
-
Nanobacteria
- NTs:
-
Neurotrophins
- PNH:
-
Paroxysmal nocturnal haemoglobinuria
- HSC:
-
Haematopoietic stem cell
- GPI:
-
Glycosylphosphatidylinositol
- PIGA:
-
Phosphatidylinositol glycan anchor biosynthesis class A gene
- CBCT:
-
Cone-beam computed tomography
References
Pettiette MT, Zhong S, Morett AJ, et al. Potential correlation between statins and pulp chamber calcification. J Endod. 2013;39:1119–23.
Sener S, Cobankara FK, Akgunlu F. Calcifications of the pulp chamber: prevalence and implicated factors. Clin Oral Investig. 2009;13:209–15.
Stanley HR, White CL, McCray L. The rate of tertiary (reparative) dentine formation in the human tooth. Oral Surg Oral Med Oral Pathol. 1966;21:180–9.
Näsström K. Dentin formation after corticosteroid treatment. A clinical study and an experimental study on rats. Swed Dent J Suppl. 1996;115:1–45.
Edds AC, Walden JE, Scheetz JP, et al. Pilot study of correlation of pulp stones with cardiovascular disease. J Endod. 2005;31:504–6.
Gold SI. Root canal calcification associated with prednisone therapy: a case report. J Am Dent Assoc. 1989;119:523–5.
Mizuno N, Shiba H, Xu W, et al. Effect of neurotrophins on differentiation, calcifification and proliferation in cultures of human pulp cells. Cell Biol Int. 2007;31:1462–9.
Ranjitkar S, Taylor JA, Townsend GC. A radiographic assessment of the prevalence of pulp stones in Australians. Austr Dent J. 2002;47:36–40.
Udoye C, Sede M. Prevalence and analysis of factors related to ooccurrence of pulp stone in adult restorative patients. Ann Med Health Sci Res. 2011;1:9–14.
Al-Nazhan S, Al-Shamrani S. A radiographic assessment of the prevalence of pulp stones in Saudi adults. Saudi Endod J. 2011;1:19–26.
Gautam S, Galgali SR, Sheethal HS, et al. Pulpal changes associated with advanced periodontal disease: a histopathological study. J Oral Maxillofac Pathol. 2017;21:58–63.
Nanjannawar GS, Vagarali H, Nanjannawar LG, et al. Pulp stone– an endodontic challenge:successful retrieval of exceptionally long pulp stones measuring 14 and 9.5 mm from the palatal roots of maxillary molars. J Contemp Dent Pract. 2012;13:719–22.
Mello-Moura AC, Bonini GA, Zardetto CG, et al. Pulp calcification in traumatized primary teeth:prevalence and associated factors. J Clin Pediatr Dent. 2011;35:383–8.
Saito T, Ogawa M, Hata Y, et al. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod. 2004;30:205–8.
Matsui S, Takahashi C, Tsujimoto Y, et al. Stimulatory effects of low- concentration reactive oxygen species on calcification ability of human dental pulp cells. J Endod. 2009;35:67–72.
Zeng J, Zhang W, Jiang H, et al. Isolation, cultivation and identification of Nanobacteriafrom dental pulp stone. Chin J Stomatol. 2006;41:498–501.
Parekh S, Kyriazidou A, BlochZupan A, et al. Multiple pulp stonesand shortened roots of unknown etiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:139–42.
Symons AL, Symons DJ. Pulpal obliteration related to long-term glucocorticosteroid medication. Spec Care Dentist. 1994;14:103–7.
Chigono Y, Daimon T, Miyagawa M, et al. Dental pulp changes observed in a patient on long-term corticosteroids. J Hard Tiss Biol. 2007;16:31–5.
Hill A, Kinoshita T, et al. Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers. 2017;18:17028–56.
Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124:2804–11.
Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–8.
Socie G, et al. Changing prognosis in paroxysmal nocturnal haemoglobinuria disease subcategories: an analysis of the International PNH Registry. Intern Med J. 2016;46:1044–53.
Yu F, Du Y, Han B. A comparative analysis of clinical characteristics of patients with paroxysmal nocturnal hemoglobinuria between Asia and Europe/America. Int J Hematol. 2016;103:649–54.
Oni SB, Osunkoya BO, Luzzatto L. Paroxysmal nocturnal hemoglobinuria: evidence for monoclonal origin of abnormal red cells. Blood. 1970;36:145–52.
Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–11.
Latour RD, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112:3099–106.
Eickstedt KW, Elsasser W. Corticotrophins and corticosteroids. In: Dukes MNG, editor. Meyler’s side effects of drugs. 11th ed. Amsterdam: Elsevier Science Publishers; 1988. p. 812–27.
Atkinson JB, Kosi M, Srikanth MS, et al. Growth hormone reduces impaired wound healing in protein-malnourished rats treated with corticosteroids. J Pediatr Surg. 1992;27:1026–8.
Anneroth G, Bloom G. Structural changes in the incisors of cortisone-treated rats. J Dent Res. 1966;45:229–35.
Ball PC. Dimensional changes in the dental tissues of the rat mandibular incisor after the systemic administration of glucocorticoid hormone. J Dent Res. 1972;51:1260.
Ball PC. The effect of adrenal glucocorticoid administration on eruption rates and tissue dimensions in rat mandibular incisors. J Anat. 1977;124(Pt 1):157–63.
Ball PC. Lack of effect of excess glucocorticoid hormone on the rate of dentin deposition in rats. J Dent Res. 1977;56:685–90.
Johannessen LB. Effects of cortisone on dentinogenesis in mandibular first molars of albino rats. Arch Oral Biol. 1964;9:421–34.
Lange A, Hammarstrom L. Cell sizes and apposition of dental hard tissues in rats. Aeta Odontol Seand. 1984;42:215–23.
Hofmman MM, Schour I. Quantitative studies in the development of the rat molar. The growth of the primary and secondary dentin from birth to 500 days of age. Anat Res. 1940;78:233–51.
Kurahashi Y, Nagai N, Watanabe K, Watanabe H, Yama K. Chronological observation of the odontogenesis of rat molars. Bull Tokyo Dent Coll. 1968;4:147–59.
Griffith JQ, Farris EJ. The Rat in Laboratory Investigation. (Scientific Books: The Rat in Laboratory Investigation). J.B. Lippincott Company, 1942; 104–65.
Cate RT. Oral histology: development, structure, and function. 1989:122–39.
Näsström K, Forsberg B, Petersson A, et al. Narrowing of the dental pulp chamber in patients with renal diseases. Oral Surg Oral Med Oral Pathol. 1985;59:242–6.
Shinozuka O, Sekiguchi G, Tamamori Y, et al. Narrowing of the dental pulp cavity in patients undergoing long-term administration of steroids. Kokubyo Gakkai Zasshi. 2001;68:294–9.
American Association of Endodontists (2010) Contemporary endodontic microsurgery: procedural advancements and treatment planning considerations. Endodontics. Colleagues for Excellence. Chicago: American Association of Endodontists.
Glendor U. Epidemiology of traumatic dental injuries—a 12 years review of the literature. Dent Traumatol. 2008;24:603–11.
Oginni AO, Adekoya-Sofowora CA. Pulpal sequelae after trauma to anterior teeth among adult Nigerian dental patients. BMC Oral Health. 2007;7:11.
Andreasen FM, Zhijie Y, Thomsen BL, Andersen PK. Occurrence of pulp canal obliteration after luxation injuries in the permanent dentition. Endod Dent Traumatol. 1987;3:103–15.
Robertson A, Andreasen FM, Bergenholtz G, Andreasen JO, Noren JG. Incidence of pulp necrosis subsequent to pulp canal obliteration from trauma of permanent incisors. J Endod. 1996;22:557–60.
Holcomb JB, Gregory WB Jr. Calcific metamorphosis of the pulp: Its incidence and treatment. Oral Surg Oral Med Oral Pathol. 1967;24:825–30.
Vinagre A, Castanheira C, Messias A, et al. Management of pulp canal obliteration-systematic review of case reports. Medicina (Kaunas). 2021;57:1237.
Oginni AO, Adekoya-Sofowora CA, Kolawole KA. Evaluation of radiographs, clinical signs and symptoms associated with pulp canal obliteration: an aid to treatment decision. Dent Traumatol. 2009;25:620–5.
Fonseca Tavares WL, Diniz Viana AC, de Carvalho MV, et al. Guided endodontic access of calcified anterior teeth. J Endod. 2018;44:1195–9.
Zubizarreta-Macho A, Valle Castano S, Montiel-Company JM. Effect of computer-aided navigation techniques on the accuracy of endodontic access cavities: a systematic review and meta-analysis. Biology. 2021;10:212.
Pinto D, Marques A, Pereira JF. Long-term prognosis of endodontic microsurgery-a systematic review and meta-analysis. Medicina. 2020;56:447.
Mainkar A. A systematic review of the survival of teeth intentionally replanted with a modern technique and cost-effectiveness compared with single-tooth implants. J Endod. 2017;43:1963–8.
Torabinejad M, Dinsbach NA, Turman M. Survival of intentionally replanted teeth and implant-supported single crowns: a systematic review. J Endod. 2015;41:992–8.
Acknowledgements
Not applicable
Funding
The work was supported by Hebei Province 333 talent funding project A202002031 and the science and technology support program of Xingtai, Hebei Province, 2019ZC145. The grant funded the design of the study and the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design of study: JD, YX, and SS. Acquisition, analysis, and interpretation of data: ZH, JK, and HW. Drafting of the manuscript: JD, HX, and HY. Revising of the manuscript: JD, YX, and SS. The published version was accepted by all writers.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethical Committee of Hebei Eye Hospital gave its approval to the investigation of the widespread pulpal obliteration in a patient with paroxysmal nocturnal hemoglobinuria. The approval number is 2022LW001.
Consent for publication
Written informed consent was obtained from the patient’s brother who is her only surviving relative for publication of this Case report and any accompanying images. The patient’s brother signed the consent to allow us to publish the case. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
It is stated by the authors that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiandong, B., Yunxiao, Z., Zuhua, W. et al. Generalized pulp canal obliteration in a patient on long-term glucocorticoids: a case report and literature review. BMC Oral Health 22, 352 (2022). https://doi.org/10.1186/s12903-022-02387-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-022-02387-9