Abstract
Background
The objective was to qualitatively and quantitatively describe the subgingival cultivable bacteria in Albanian subjects and to compare it with a similar Spanish population.
Materials and methods
Consecutive patients, diagnosed as periodontitis in stages I–II or III–IV, and as periodontally healthy or with gingivitis, were studied clinically and microbiologically by means of microbiological culture, including total anaerobic counts, proportions, and frequency of detection of target species. Outcome variables were analysed by Mann–Whitney, Kruskal–Wallis, ANOVA, ANCOVA and Chi-square tests.
Results
In this cross-sectional study, 83 (Albania) and 90 (Spain) subjects were included. No statistically significant differences were observed between test and control populations regarding demographic variables or smoking habit. Significantly higher total anaerobic counts in the Albanian population (p = 0.022) were observed, especially in the periodontal health/gingivitis group (p = 0.001). In the test population, the proportions of the cultivable bacteria of Fusobacterium nucleatum were significantly lower in both the healthy/gingivitis (p = 0.022) and stages I–II periodontitis (p = 0.034) groups.
Conclusions
The subgingival cultivable bacteria in both periodontitis and non-periodontitis subjects from Albania showed significantly higher total anaerobic counts and lower proportions of the cultivable bacteria of F. nucleatum than a similar population of subjects from Spain.
Similar content being viewed by others
Background
Periodontal diseases are chronic inflammatory conditions affecting the tooth supporting tissues caused by dental biofilms, but modulated by different patient and environment related risk factors. The pathogenesis of both gingivitis and periodontitis result from an imbalance between the infectious challenge (bacterial pathogens organized in dental biofilms) and the host response [1, 2]. Systemic risk factors will influence the host response by the genetic predisposition and systemic health status of the subject, while the environmental conditions as well as by lifestyle factors may influence both the subgingival microbiota and the host response [2]. For example, there is strong evidence that tobacco smoking is a relevant risk factor for the onset and progression of periodontitis, what contributes to the higher prevalence and severity of periodontitis in smokers [3, 4]. Nutrition has also been reported as a relevant lifestyle factor in the onset and progression of periodontal diseases, although its role as a true risk factor has not yet been established [5].
Environmental and lifestyle factors vary among countries and subject populations from different geographical areas and these changes may influence the aetiology and progression of periodontal diseases in these specific places. In fact, differences in the composition of the subgingival microflora have been demonstrated when sampling subjects with similar clinical characteristics, but belonging to different geographical populations [6]. Whereas it is well established that periodontal pathogens such as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia have demonstrated a strong association with periodontitis [1], and their relative counts and proportions may be different according to different geographical areas [7, 8]. For example, A. actinomycetemcomitans, [9] was reported in high prevalence in the Chinese population, compared to that of European and North American populations. Similarly, in Brazil [10] A. actinomycetemcomitans was found in high numbers in both periodontitis and in healthy subjects. In Morocco, subjects diagnosed of periodontitis demonstrated a significantly higher prevalence of A. actinomycetemcomitans (35.6%) [11], compared to only 5.7% in periodontitis patients in Spain [12].
These studies therefore suggest that differences in the subgingival microbiota composition may occur in populations with different environmental and lifestyle conditions. In fact, differences in the profiles of the subgingival microbiota were also reported by our research group when comparing periodontitis subjects from Spain and The Netherlands [8], and when comparing periodontitis subjects from Colombia, Chile and Spain [13], or more recently, even comparing subjects between Colombia and Spain [14]. Other authors have postulated that beyond the environmental and lifestyle factors, the genetic background of the subjects may also influence the composition of the subgingival microbiota [6, 15]. In fact, it has been reported ethnicity-specific subgingival microbiomes when comparing two populations sharing a common environment but different genetic background [16].
Albania is a developing country in the western Balkans surrounded by Montenegro, Kosovo, Macedonia and Greece, with a population of approximately 3.1 million people. The drastic change in economic and social political conditions in the last 2 decades has significantly impacted on the socioeconomic and environmental factors that may have an impact on health [17], what may also influence the prevalence and severity of periodontal diseases in this country [18]. Factors such as low socioeconomic status, lack of dental health education and limited access to proper oral-health care in a highly diverse ethnic population may influence the prevalence of periodontal diseases by affecting the composition of the oral microbiota.
Since there are no studies characterising the subgingival microbiota from Albanian subjects, we have designed this case–control study, where consecutive subjects were periodontally diagnosed with the new classification of periodontal diseases [19, 20], and the composition of their subgingival microbiota has been studied using anaerobic culture microbiology. As controls, we have characterised, both clinically and microbiologically, a similar population of Spanish subjects. The working hypothesis is that the clear environmental differences between Albania and Spain will significantly influence the microbial composition of the subgingival cultivable bacteria in subjects with different periodontal health status.
Materials and methods
Study design
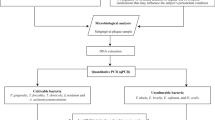
This study was designed as a cross-sectional observational study and it is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [21]. The protocol of this investigation was approved by the local ethical committees in the respective countries (references 18/128-E in Spain and 197 in Albania) and considers all aspects of the Helsinki Declaration regarding experimentation involving human.
Patient sample
Subjects seeking dental attending the Faculty of Dentistry in Tirana (Albanian University, Albania) and the Faculty of Dentistry in Madrid [University Complutense of Madrid (UCM), Spain] were screened between April–May 2018 and March 2020 and registered the socio-demographic characteristics of the patients, such as age, gender, origin, smoking, systemic health, medications, and conditions. Patients included in this study met the following criteria: (1) age between ≥ 30 and ≤ 60. The following exclusion study criteria were also evaluated: (1) having less than 16 teeth; (2) patient with a periodontal abscess or necrotizing periodontal diseases; (3) use of systemic antibiotics in the previous month; (4) patient with relevant systemic diseases (diabetes, polymorphonuclear neutrophil defects, other immune system disorders); (5) pregnant or lactating patient; and (6) patient with current anti-inflammatories, anticonvulsant, calcium channel blockers, or immunosuppressant treatments, or 6 months prior to the sample.
When subjects fulfilled these criteria, they were verbally informed about the study and were asked to participate by signing an informed consent. Upon acceptance, each patient was appointed for the study visits.
Clinical and radiological examination
The patients received a complete periodontal and radiographic examination, including gingival recession, probing depth (PD), clinical attachment loss (CAL) and bleeding on probing (BoP) [22] using a UNC-15 periodontal probe (HuFriedy, Leinmen, Germany). Plaque index (PlI) [23] was evaluated after rinsing the patient with a plaque disclosing solution containing erythrosine (Plac-Control®, Dentaid, Barcelona, Spain).
After this visit, the included subjects were segmented by their periodontal status in three categories using the following criteria [19, 20]: (1) periodontal health and gingivitis: no CAL, no radiographic bone loss (RBL) and PD ≤ 3 mm, assuming no pseudo-pockets; (2) stages I and II periodontitis: PD 4–5 mm, mostly horizontal RBL and no tooth loss due to periodontal reasons. CAL will be 1–2 mm (stage I) or 3–4 mm (stage II), while RBL affects only the coronal third (< 15% for stage I and 15–33% for stage II); and (3) stages III and IV periodontitis: At least two non-adjacent sites with CAL ≥ 5 mm or reaching the middle third of the root, with PD ≥ 6 mm. Evidence of tooth loss due to periodontal reasons.
Microbiological procedures
Microbiological sampling
Samples were taken with two consecutive standardized 30# sterile paper points (Maillefer, Ballaigues, Switzerland). Paper points were inserted into the crevice or pocket and left in place for 10 s. Prior to sampling, four sites per patient were selected, one in each quadrant. The selected sites were isolated from saliva and supragingival plaque contamination with the use of cotton rolls and compressed air. In periodontal health/gingivitis subjects, subgingival samples were taken from the mesio-buccal sites of the first molars and, when absent, from the adjacent second molars (the next alternative was the second premolars and from there, any teeth present mesially). In subjects with periodontitis, subgingival samples were taken from the most accessible site with the deepest PD and BoP, per quadrant. The eight paper points were transferred into a screw-capped vial, containing 1.5 ml of reduced transport fluid (RTF) [24], so an individual pooled sample was obtained from each patient. Samples were sent directly (Spanish samples) or via courier (Albanian samples) to the Laboratory of Research at UCM, Spain, where they were processed within 24–36 h. RTF was the ideal transport medium, as it has been shown to maintain a good viability of anaerobes up to four days after sample collection [24].
Direct anaerobic culture
At the Laboratory, samples were homogenized by vortexing for 30 s, and serially diluted in phosphate buffer saline (PBS) (dilutions 10–1, 10–2, 10–3 and 10–4). For each sample, 100 µl of at least two of the dilutions were plated on non-selective blood agar medium (Blood Agar Base II, Oxoid, Basingstoke, England), supplemented with haemin (5 mg/l), menadione (1 mg/l) and 5%, sterile horse blood. Plates were incubated for up to 14 days in anaerobic conditions (80% N2, 10% CO2 and 10% H2) at 37 °C. After 7–14 days of anaerobic incubation, suspected colonies were further identified by microscopy, gram-staining and enzyme activity (see Additional file 1: Table S1). The counts of representative colonies (those with colony morphologies compatible with target pathogen morphology) were carried out.
For isolation and quantification of A. actinomycetemcomitans, another 100 µl of the 10–1 dilution of each sample and 100 µl without dilution were plated onto the selective medium Dentaid-1 [25], that was incubated for 3 days in air with 5% CO2 at 37 °C.
Data analysis
Sample size calculation
The outcome variable “proportion of the anaerobic cultivable bacteria of P. gingivalis” was selected to calculate the sample size. With a proportion of P. gingivalis in Spain of 22.21% [13] and in order to detect a difference in proportions of 16.72% between Albania and Spain, with a 90% of power and a significance of 95%, at least 88 patients per country were necessary. Besides, and to narrow differences between different age groups in different conditions, the overall sample was a uniformed stratified sampling, in which the same size for all the defined categories were assigned. The desired sampling distribution was 30 patients in each category (periodontal health/gingivitis, stages I–II periodontitis, stages III–IV periodontitis), and 10 patients per age cohort, 30–40 years, 41–50 years, 51–60 years, within each periodontal status category.
Statistical analysis
The statistical unit of the study was the patient. For continuous data, Kolmogorov–Smirnov test and distribution of data were used to assess normality. Data were expressed as means and standard deviations (SD), and as median and interquartile ranges (IQR) for non-parametric data. Categorical data were expressed as percentages.
Demographic data and clinical variables were analysed by Student t test, ANOVA test and chi-square test with probability values adjusted with the Bonferroni correction. For microbiological outcome variables, total anaerobic counts were calculated on blood-agar plates and expressed in total colony-forming units/ml (CFU/ml). Counts for each specific bacterial species, as well as their percentage of total cultivable bacteria, were also calculated for each patient. Counts and proportions were calculated considering all samples. The logarithmic transformation of CFU of bacterial counts was designed to normalise the data distribution. Microbiological variables were compared by t test and ANOVA test, for parametric data, and U Mann–Whitney test or Kruskal–Wallis test with Dunn–Bonferroni post hoc tests, for non-parametric data. Differences between the two countries were further explored by analysis of covariance (ANCOVA), with country as the factor, and PlI and PD were entered into the model as co-variates. In this case, ANCOVA model adjusted means and confidence intervals (CI) were calculated. Proportions of target pathogens were log-transformed to achieve homogeneity of variances.
For categorical data, chi-square test was used, with Bonferroni correction for multiplicity when was necessary.
All statistical analyses were performed using SPSS 20 program package (SPSS Inc, Chicago, IL, USA) and the level of significance was set in 0.05.
Results
A total of 186 patients were initially recruited (98 in Spain and 88 in Albania). Eight subjects in Spain and five in Albania were excluded due to technical issues in sample transportation and bacteria culturing. A total of 173 subjects, 90 in Spain and 83 in Albania, were recruited, with a similar distribution within the pre-established categories: periodontal health/gingivitis (55 in total, 30 in Spain and 25 in Albania), stages I–II periodontitis (58, 30 and 28, respectively) and stages III–IV periodontitis (60, 30 and 30, respectively). As depicted in Table 1, there were no statistically significant differences between these populations, either for age and gender or among the periodontal categories. Similarly, the percentage of smokers was not significantly different.
Table 2 depicts the clinical variables in the recruited patients within the three established categories. Overall, subjects from Spain showed a statistically significant higher mean PD (p = 0.025). PD was significantly higher in Spanish subjects in stages III–IV periodontitis (p < 0.001) and PlI in the periodontal health/gingivitis group (p < 0.001). Conversely, PlI was significantly higher in stages III–IV periodontitis (p = 0.036) for Albanian population.
Table 3 presents the detection of different bacterial species in subgingival samples of Albanian population. The most prevalent bacterial species in periodontal health/gingivitis, stages I–II and stages III–IV periodontitis groups were Fusobacterium nucleatum (92%, 92.9% and 86.7%, respectively), P. gingivalis (68%, 82.1% and 80%, respectively), Prevotella intermedia (52%, 71.4% and 76.7%, respectively) and Eikenella corrodens (46.7% in stages III–IV). Statistically significant differences between subjects with and without periodontitis were only found for P. intermedia (p = 0.048) and E. corrodens (p = 0.035). In addition, E. corrodens was detected with a higher frequency in stages III–IV than in periodontal health/gingivitis (p = 0.016). In terms of counts and proportions, comparing subjects with and without periodontitis, statistically significant differences were detected for P. gingivalis (p = 0.002 for counts and p = 0.016 for proportions), P. intermedia (p = 0.002 and p = 0.020, respectively) and E. corrodens (p = 0.014 and p = 0.043, respectively). In the analysis by periodontal status, significant differences were only observed in counts for P. gingivalis (p = 0.005), P. intermedia (p = 0.005) and E. corrodens (p = 0.020), between periodontal health/gingivitis and stages III–IV periodontitis. On the other hand, statistically significant higher counts and proportions of Actinomyces odontolyticus were detected in patients without periodontitis (p = 0.030 and p = 0.030, respectively), but such differences were not maintained in the analysis by periodontal status.
Table 4 depicts the total anaerobic count results of the test and control group analysed together, and by periodontal status and country. Significantly higher anaerobic counts were detected in the periodontitis categories (stages I–II (p < 0.001) and III–IV (p < 0.001)), compared with the periodontal health/gingivitis group. Higher total counts were observed in Albanian subjects (p = 0.022), being this difference statistically significant in the periodontal health/gingivitis group (p = 0.001).
Tables 5, 6 and 7 depict the subgingival cultivable bacteria (target periodontal pathogens) in both country populations segmented by the three categories. Samples from Albanian subjects were characterized by significantly lower proportions of F. nucleatum and P. intermedia. In periodontal health/gingivitis and in stages I–II periodontitis, F. nucleatum was present in significantly lower proportion (p = 0.022 and p = 0.034, respectively) in the Albanian samples. The same was true in the category stages III–IV periodontitis for P. intermedia (p = 0.038). However, when the proportions of P. intermedia were adjusted for plaque index and probing depth, as possible confounding factors between countries by an ANCOVA model, this difference between countries was no longer observed (Table 8).
No statistically significant differences were found between the countries for counts or frequencies of detection of the target pathogens, although some tendencies were observed. In stages I–II periodontitis, samples from Albanian subjects presented less frequently P. gingivalis (82.1% versus 96.7%, p = 0.097) and P. intermedia (71.4% versus 90.0%, p = 0.071). Similar findings were observed for T. forsythia in stages III–IV periodontitis (20.0% versus 43.3%, p = 0.052). Conversely A. actinomycetemcomitans (10.0% versus 0%), and E. corrodens (76.7% versus 23.3%, p = 0.058) were present more frequently in the Albanian population, in stages III–IV periodontitis.
Table 9 depicts the counts and frequencies of detection of target pathogens by clinical categories. Lower counts, proportions, and frequencies of detection of P. gingivalis and P. intermedia were detected in the periodontal health/gingivitis group, compared with stages I–II (p ≤ 0.05) or stages III–IV periodontitis (p ≤ 0.05) groups. Similarly, T. forsythia was detected in lower counts, proportions, and frequencies of detection in the periodontal health/gingivitis group, compared with stages I–II periodontitis (p ≤ 0.05); F. nucleatum was detected in lower counts in periodontal health/gingivitis group when compared with stages I–II (p = 0.012) or stages III–IV periodontitis (p = 0.001); and E. corrodens was detected in lower counts in periodontal health/gingivitis when compared with stages III–IV periodontitis (p = 0.028). For Parvimonas micra, statistically significant higher frequencies of detection were observed in the periodontal health/gingivitis group when compared with stages I–II periodontitis (p = 0.045).
Discussion
The present study evaluated two similar populations in terms of age, gender and smoking habits, but from two countries with different environments (Albania and Spain). These subjects have provided microbiological samples processed by anaerobic culturing in a single laboratory using the same microbiological diagnostic technology. These recruited subjects were present in similar numbers in the three diagnostic categories, with minimal differences in the clinical parameters (deeper mean PD in Spanish subjects within the stages III–IV periodontitis category, and lower plaque index levels in Albanian subjects in the periodontal health/gingivitis group but higher in the stage III–IV periodontitis). The analysis of the subgingival cultivable bacteria of these two distinct populations has shown that Albanian subjects presented higher anaerobic counts, especially in periodontal health/gingivitis subjects, and lower proportions of F. nucleatum in periodontal health/gingivitis and stages I–II periodontitis.
The fact that all samples were processed by the same microbiological laboratory may reduce the differences due to sample processing reported in previous studies with similar objectives [6, 8, 11, 13, 14]. Also, the fact that the clinical parameters within the pre-established clinical categories were based on the diagnostic criteria of the new classification of periodontal diseases [19, 20] may have reduced the likely differences in the subgingival bacteria due to the differences in periodontal status. Although the Spanish patients showed deeper mean PDs and lower PlI levels in stages III–IV periodontitis, and Albanian patients presented a lower PlI in the periodontal health/gingivitis group, these differences may be anecdotal and with a clear lack of clinical significance, except in stages III–IV periodontitis.
In the present study, when evaluating the microbiological profile of the Albanian population, the bacterial species with the highest frequency of detection in periodontitis were P. gingivalis, P. intermedia, F. nucleatum and E. corrodens. There is no available information from Albania to compare with the findings. If they are compared with findings from other geographical locations (Spain, Morocco, Colombia, Chile), P. gingivalis, P. intermedia and F. nucleatum are consistently three of the most frequently detected bacterial species in subgingival samples in patients with periodontitis by means of culture techniques [8, 11, 13, 14]. E. corrodens has also shown higher frequencies in periodontitis than in periodontally healthy subjects [26]. However, in the Albanian population studied, only P. intermedia and E. corrodens showed a statistically significantly higher prevalence in periodontitis than in subjects without periodontitis, which may suggest that P. gingivalis and F. nucleatum are also frequently present in subjects without periodontitis in Albania.
When the Albanian subjects were compared to a similar Spanish population, two main differences were identified, namely total anaerobic counts and the proportions of specific target species. For the differences in total anaerobic counts, statistically significant differences were detected in the whole sample, which corresponds to higher total counts in Albanian patients than in Spanish patients in the group of subjects with periodontal health or gingivitis. This finding is consistent with a previous study observing microbiological differences in subjects according to race/ethnicity, family income or education, as well as smoking, diet and health habits, or access to dental care [27]. While smoking is not a differentiating variable in the present study, socio-economic and/or socio-demographic differences might have influenced the results.
For differences in the proportions of specific target species, significantly lower proportions of F. nucleatum, in periodontal health/gingivitis and in stages I–II periodontitis, were detected in Albanian samples. This finding is in line with a study showing that subjects with low socio-economic status and low levels of oral diseases (caries and/or periodontitis) have lower amounts of certain members of the Fusobacterium genus [28]. It is unclear whether these differences may reflect that dysbiotic biofilms in Albanian patients were not clearly associated with specific pathogens, while the corresponding dysbiotic biofilms in Spanish subjects were associated with specific pathogens. Previous studies with populations in Spain have highlighted the possible relevant role of P. gingivalis [8, 13, 14], what may support the importance of this pathogen as a key-stone pathogen responsible of the bacterial dysbiosis concept [29].
Although not statistically significant, other microbiological differences also showed clear tendencies in terms of frequencies of detection, depicting higher prevalence of A. actinomycetemcomitans and E. corrodens in Albania, and higher prevalence of P. gingivalis, P. intermedia and T. forsythia in Spain. These findings support previous reports comparing the subgingival cultivable bacteria of Colombian and Spanish patients [14], that suggests that dysbiotic biofilms could be associated with larger amounts of microorganisms in Albanian subjects, while in Spain the impact of key pathogens may be more relevant. In addition, the role of A. actinomycetemcomitans in promoting dysbiosis of in a limited number of Albanian patients should also be considered, which is consistent with the results of different studies on Eastern Europe populations [30,31,32], as compared with lower levels in Spain [8, 12,13,14].
When evaluating the microbiological findings within the different periodontal categories, differences were observed in terms of total anaerobic counts, and in counts, proportions and frequencies of detection of target bacterial species, including the most relevant periodontal pathogens, P. gingivalis, T. forsythia, P. intermedia and F. nucleatum, what is in agreement with previous studies using other classification systems [6, 8, 31] or with studies using the same 2018 classification [14, 32]. Whether these differences are causal or secondary to differences in PDs cannot be explored in a cross-sectional study [33].
The present study used culture techniques for the identification of the cultivable bacteria associated with periodontitis. While Next Generation Sequencing (NGS) approaches are currently frequently used, an initial characterization of a previously not tested population (as Albanian subjects) may benefit from a simpler approach. However, the value of culture techniques should not be underestimated, alone or in combination with other approaches, since it has been considered that it is important to have parallel culture libraries [34, 35], that benefits from the improvement of microbiological culturing, e.g. with the introduction of more competent anaerobic handling and incubation procedures, so culture is reinvented every day [36]. Thus, many other researchers still believe that cultivation continues to be an interesting alternative for microbiological testing [37] and its use allows for appropriate comparisons with previous studies that have also used culture techniques.
The results of the present study should be interpreted with caution due to the clear limitations of the microbiological methodology used, e.g. the microbiological samples from Albania were sent by courier to Spain, and although the same standardised approach to sampling was followed in both centres [38], and the time interval between sampling and plating was the same for both countries (24–36 h), it cannot be discarded that the ideal transport conditions might not have been maintained for some samples, which could have impacted on the viability of some microorganisms [24]. Another limitation is associated with the sampling strategy, since only the four deepest sites were sampled in each patient, which may underestimate detection frequencies [39]; however, this strategy was validated in the early nineties [40, 41] and it has been extensively used in periodontal microbiology. Moreover, the relatively small sample size without providing information about other possible sources of bias (as differences in socio-economic status) may have limited the opportunity to find significant differences. Finally, culture techniques are not able to provide a thorough research of the subgingival microbiota, thus further in-depth analysis, e. g. using NSG approaches, would be necessary to have a more comprehensive picture of the whole microbiota of the Albanian population, including non-culturable bacterial species.
Conclusions
Within the limitations of the present study, it can be concluded that the microbiological profile of the subgingival cultivable bacteria in periodontitis and non-periodontitis patients has demonstrated statistically significant differences between Albanian and Spanish patients, with higher total anaerobic counts in Albania and higher proportions of cultivable bacteria of F. nucleatum in Spain.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to data protection regulations and ethical concerns but are available from the corresponding author on reasonable request.
Abbreviations
- UCM:
-
University Complutense of Madrid
- PD:
-
Probing depth
- CAL:
-
Clinical attachment loss
- BoP:
-
Bleeding on probing
- PlI:
-
Plaque index
- RBL:
-
Radiographical bone loss
- RTF:
-
Reduced transport fluid
- PBS:
-
Phosphate buffer saline
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- CFU:
-
Colony-forming units
- CI:
-
Confidence interval
- n:
-
Sample size
- n (%):
-
Number and percentage of positive samples
References
Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontology. 2000;2002(28):12–55.
Amaliya A, Laine ML, Delanghe JR, Loos BG, Van Wijk AJ, Van der Velden U. Java project on periodontal diseases: periodontal bone loss in relation to environmental and systemic conditions. J Clin Periodontol. 2015;42(4):325–32.
Tonetti MS. Cigarette smoking and periodontal diseases: etiology and management of disease. Ann Periodontol. 1998;3(1):88–101.
Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70(1):30–43.
Van der Velden U, Kuzmanova D, Chapple IL. Micronutritional approaches to periodontal therapy. J Clin Periodontol. 2011;38(Suppl 11):142–58.
Haffajee AD, Bogren A, Hasturk H, Feres M, Lopez NJ, Socransky SS. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J Clin Periodontol. 2004;31(11):996–1002.
Cao CF, Aeppli DM, Liljemark WF, Bloomquist CG, Bandt CL, Wolff LF. Comparison of plaque microflora between Chinese and Caucasian population groups. J Clin Periodontol. 1990;17(2):115–8.
Sanz M, van Winkelhoff AJ, Herrera D, Dellemijn-Kippuw N, Simón R, Winkel E. Differences in the composition of the subgingival microbiota of two periodontitis populations of different geographical origin. A comparison between Spain and The Netherlands. Eur J Oral Sci. 2000;108(5):383–92.
Mombelli A, Gmür R, Lang NP, Corbert E, Frey J. Actinobacillus actinomycetemcomitans in Chinese adults. Serotype distribution and analysis of the leukotoxin gene promoter locus. J Clin Periodontol. 1999;26(8):505–10.
Colombo AP, Teles RP, Torres MC, Souto R, Rosalém WJ, Mendes MC, Uzeda M. Subgingival microbiota of Brazilian subjects with untreated chronic periodontitis. J Periodontol. 2002;73(4):360–9.
Chahboun H, Arnau MM, Herrera D, Sanz M, Ennibi OK. Bacterial profile of aggressive periodontitis in Morocco: a cross-sectional study. BMC Oral Health. 2015;15:25.
Mínguez M, Pousa X, Herrera D, Blasi A, Sánchez MC, León R, Sanz M. Characterization and serotype distribution of Aggregatibacter actinomycetemcomitans isolated from a population of periodontitis patients in Spain. Arch Oral Biol. 2014;59(12):1359–67.
Herrera D, Contreras A, Gamonal J, Oteo A, Jaramillo A, Silva N, Sanz M, Botero JE, León R. Subgingival microbial profiles in chronic periodontitis patients from Chile, Colombia and Spain. J Clin Periodontol. 2008;35(2):106–13.
Pianeta R, Iniesta M, Castillo DM, Lafaurie GI, Sanz M, Herrera D. Characterization of the subgingival cultivable microbiota in patients with different stages of periodontitis in Spain and Colombia. A cross-sectional study. Microorganisms. 2021;9(9):1940.
Kilian M, Frandsen EV, Haubek D, Poulsen K. The etiology of periodontal disease revisited by population genetic analysis. Periodontology. 2000;2006(42):158–79.
Mason MR, Nagaraja HN, Camerlengo T, Joshi V, Kumar PS. Deep sequencing identifies ethnicity-specific bacterial signatures in the oral microbiome. PLoS One. 2013;8(10):e77287.
Nuri B. Heath care systems in transition: Albania. In: Tragakes E, editor. Health care systems in transition, vol. 4. Copenhagen: European Observatory on Health Care Systems; 2002.
König J, Holtfreter B, Kocher T. Periodontal health in Europe: future trends based on treatment needs and the provision of periodontal services–position paper 1. Eur J Dent Educ. 2010;14(Suppl 1):4–24.
Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, Geisinger ML, Genco RJ, Glogauer M, Goldstein M, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S74–84.
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S173–82.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9.
Badersten A, Nilvéus R, Egelberg J. Effect of non-surgical periodontal therapy. VI. Localization of sites with probing attachment loss. J Clin Periodontol. 1985;12(5):351–9.
O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43(1):38.
Syed SA, Loesche WJ. Survival of human dental plaque flora in various transport media. Appl Microbiol. 1972;24(4):638–44.
Alsina M, Olle E, Frias J. Improved, low-cost selective culture medium for Actinobacillus actinomycetemcomitans. J Clin Microbiol. 2001;39(2):509–13.
Botero JE, Contreras A, Lafaurie G, Jaramillo A, Betancourt M, Arce RM. Occurrence of periodontopathic and superinfecting bacteria in chronic and aggressive periodontitis subjects in a Colombian population. J Periodontol. 2007;78(4):696–704.
Renson A, Jones HE, Beghini F, Segata N, Zolnik CP, Usyk M, Moody TU, Thorpe L, Burk R, Waldron L, et al. Sociodemographic variation in the oral microbiome. Ann Epidemiol. 2019;35:73-80 e72.
Belstrom D, Holmstrup P, Nielsen CH, Kirkby N, Twetman S, Heitmann BL, Klepac-Ceraj V, Paster BJ, Fiehn NE. Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. J Oral Microbiol. 2014;6:23609.
Hajishengallis G, Diaz PI. Porphyromonas gingivalis: immune subversion activities and role in periodontal dysbiosis. Curr Oral Health Rep. 2020;7(1):12–21.
Sakellari D, Katsikari A, Slini T, Ioannidis I, Konstantinidis A, Arsenakis M. Prevalence and distribution of Aggregatibacter actinomycetemcomitans serotypes and the JP2 clone in a Greek population. J Clin Periodontol. 2011;38(2):108–14.
Doğan B, Chen J, Çiftlikli SY, Huang J, Kadir T, Alnıak AK, Chen C. Occurrence and serotype distribution of Aggregatibacter actinomycetemcomitans in subjects without periodontitis in Turkey. Arch Oral Biol. 2016;61:125–9.
Tomšič K, Rodič K, Sotošek A, Videmšek P, Seme K, Herrera D, Sanz M, Gašperšič R. Do differences in cultivable subgingival species exist between different periodontitis stages and grades? Oral Health Prev Dent. 2021;19(1):15–24.
Ge X, Rodriguez R, Trinh M, Gunsolley J, Xu P. Oral microbiome of deep and shallow dental pockets in chronic periodontitis. PLoS One. 2013;8(6):e65520.
Donachie SP, Foster JS, Brown MV. Culture clash: challenging the dogma of microbial diversity. ISME J. 2007;1(2):97–9.
Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A. Lessons learned and unlearned in periodontal microbiology. Periodontol 2000. 2013;62(1):95–162.
Contreras A, Moreno SM, Jaramillo A, Pelaez M, Duque A, Botero JE, Slots J. Periodontal microbiology in Latin America. Periodontol 2000. 2015;67(1):58–86.
Tanner AC. Anaerobic culture to detect periodontal and caries pathogens. J Oral Biosci. 2015;57(1):18–26.
Casas A, Herrera D, Martin-Carnes J, Gonzalez I, O’Connor A, Sanz M. Influence of sampling strategy on microbiologic results before and after periodontal treatment. J Periodontol. 2007;78(6):1103–12.
Haffajee AD, Socransky SS. Effect of sampling strategy on the false-negative rate for detection of selected subgingival species. Oral Microbiol Immunol. 1992;7(1):57–9.
Mombelli A, McNabb H, Lang NP. Black-pigmenting gram-negative bacteria in periodontal disease. I. Topographic distribution in the human dentition. J Periodontal Res. 1991;26(4):301–7.
Mombelli A, McNabb H, Lang NP. Black-pigmenting gram-negative bacteria in periodontal disease. II. Screening strategies for detection of P. gingivalis. J Periodontal Res. 1991;26(4):308–13.
Acknowledgements
We thank for their technical assistance Maria Sanchez and Ana O´Connor, from the Laboratory of Research, University Complutense of Madrid, as well as the Albanian University.
Funding
This work was partially supported by Cátedra Extraordinaria Dentaid de Investigación en Periodoncia, University Complutense of Madrid, Spain.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.S. and D.H.; patient recruitment and sampling, G.T. and M.I.; microbiological analyses, M.I.; validation, M.I., M.S., and D.H.; formal analysis, M.I. and D.H.; writing—original draft preparation, G.T., M.I., M.S. and D.H.; writing—review and editing G.T., M.I., M.S. and D.H.; supervision, M.I. and D.H.; project administration, M.I. and D.H.; funding acquisition, M.S. and D.H.. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors confirm that the study has been approved by the Clinical Research Ethical Committee of Hospital Clínico San Carlos in Madrid, Spain (reference number 18/128-E) and by the Ethics Committee of Albanian University (reference number 197) and considers all aspects of the Helsinki Declaration regarding experimentation involving human. Informed consents were obtained from all included participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Presumptive identification of bacterial species in culture.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tafaj, G., Iniesta, M., Sanz, M. et al. The subgingival cultivable bacteria of Albanian subjects with different periodontal status compared to a similar population of Spanish subjects: a case control study. BMC Oral Health 22, 89 (2022). https://doi.org/10.1186/s12903-022-02121-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-022-02121-5