Abstract
Background
Oral squamous cell carcinoma (OSCC) is associated with a poor 5-year survival rate. In general, patients diagnosed with small tumors have a fairly good prognosis, but some small tumors have an aggressive behavior leading to early death. There are at present no reliable prognostic biomarkers for oral cancers. Thus, to optimize treatment for the individual patient, there is a need for biomarkers that can predict tumor behavior.
Method
In the present study the potential prognostic value of plectin was evaluated by a tissue microarray (TMA) based immunohistochemical analysis of primary tumor tissue obtained from a North Norwegian cohort of 115 patients diagnosed with OSCC. The expression of plectin was compared with clinicopathological variables and 5 year survival.
Results
The statistical analysis revealed that low expression of plectin in the tumor cells predicted a favorable outcome for patients with non-metastatic disease (p = 0.008). Furthermore, the expression of plectin was found to correlate (p = 0.01) with the expression of uPAR, which we have previously found to be a potential prognostic marker for T1N0 tumors.
Conclusions
Our results indicate that low expression of plectin predicts a favorable outcome for patients with non-metastatic OSCC and the expression level of plectin may therefore be used in the treatment stratification for patients with early stage disease.
Similar content being viewed by others
Background
Squamous cell carcinoma (SCC) accounts for more than 90 % of the malignant neoplasms of the head and neck region [1, 2]. Head and neck SCC (HNSCC) have a 5-year survival rate of approximately 50 %, [3, 4] and are ranked as the eighth leading cause of cancer death worldwide [5].
OSCC includes tumors located in the mobile tongue, floor of the mouth, buccal mucosa, gingival rim and the hard and soft palate. The most widely used classification-system of OSCC is the TNM-system by the Union for International Cancer Control [6]. This system is used to describe the anatomical extent of the disease at diagnosis based on primary tumor size and extent of tumor growth (T), regional lymph node metastasis (N) and distant metastasis (M), and is the basis for stage grouping these patients [7, 8]. By morphological assessment, tumors are graded as well, moderate and poorly differentiated [1]. The TNM-classification, and to a lesser extent, the grade of differentiation, are often used as predictors for outcome. However, OSCCs are molecularly heterogeneous and tumors with identical TNM-classification and differentiation grade may differ in aggressiveness and response to treatment [7, 9]. This unpredictable behavior raises the need for a diagnostic tool that can provide more reliable prognostic information.
A risk model based on histological analysis of the tumors has been proposed [10–12]. However, in a recent study this model did not predict the outcome of patients with tongue SCC [13]. Several previous reports have proposed different biomarkers, such as p53, EGFR, Ki67 and E-cadherin as prognostic markers for OSCC, [14–16] though none have been implemented in clinical practice. In an attempt to find new and better prognostic markers we have in this study focused on plectin Plectin is a large intracellular cytoskeletal linker protein (>500-kDa) that has been found to be important in cytoskeletal network organization. It is expressed in normal skin and in the epithelial lining of the gastrointestinal-tract, muscle cells and endothelial cells of vessels, [17] as well as in cancer arising in the esophagus, stomach, lung, pancreas and the oral cavity [17, 18]. Plectin is localized at the inner side of the plasma membrane where it is associated with intermediate filaments (IF), microtubules and microfilaments [19]. In hemidesmosomes, plectin interacts with the cytoplasmatic tail of the integrin β4 subunit. Defects in the plectin gene have been found in the severe, hereditary, skin blistering hereditary disease epidermolysis bullosa simplex, emphasizing the importance of the protein in normal epithelial cells [20]. Plectin affects mechanical, as well as dynamic properties of the cytoskeleton, and (at least in keratinocytes) plectin-deficiency has been shown to result in increased migration rates probably through activation of the Erk1/2 pathway [21]. In a study investigating HNSCC, Katada et al. found that the survival rate of patients with high plectin expression in their cancer cells was significantly decreased, and the frequency of recurrences significantly increased, compared to patients with low plectin expression [22].
In a recent study we showed that the urokinase plasminogen activator receptor (uPAR) and plasminogen activator inhibitor-1 (PAI-1) may be prognostic markers in early stage OSCC [23]. uPAR is a constituent of the plasminogen activator (PA) system, and both urokinase plasminogen activator (uPA) and uPAR are linked to increased proteolytic activity and migration of cancer cells. uPA converts plasminogen into the active serine protease plasmin, a broad spectrum protease that can degrade many different types of extracellular matrix proteins in addition to activate latent growth factors and matrix metalloproteases [24, 25]. By binding of uPA to uPAR, cancer cells can direct the proteolytic activity to the cell surface [26]. As with plectin, the increased expression of uPAR has been linked to the phenomenon epithelial-mesenchymal transition (EMT) [27–29].
In this TMA-based study, low expression of plectin was found to be a marker for a favorable prognosis in non-metastatic OSCC. Plectin and uPAR showed similar staining patterns in the tumors and there was a significant correlation between plectin and uPAR expression.
Methods
Specimens
This was a retrospective study using formalin fixed, paraffin embedded tumor samples from 115 patients with histologically verified primary OSCC in the period 1986–2002 collected from the archives of the Department of Clinical Pathology, University Hospital of North Norway. To secure a homogenous cohort, tumors with verrucous growth patterns as well as tumors from patients with previous cancer, or prior radiotherapy to the head and neck area were excluded. All patients presented with primary disease located in the oral cavity, including mobile tongue, floor of the mouth, buccal mucosa, gingiva and hard and soft palate. Relevant clinical data and TNM-classification were retrieved from patients’ files, including pathology reports, Statistics of Norway and the Cause of Death Registry. The N and M statuses were determined by clinical and radiological examination. The normal tongue mucosa tissue was obtained from tissue adjacent to the tumor tissue. The study was approved by the Regional Committees for Medical and Health Research Ethics, Northern Norway (No. 22/2007). The patient information was de-identified prior to analysis. The ethics committee deemed it unnecessary to obtain written or oral consent from the participating patients.
Tissue microarray (TMA)
A morphologically representative region of each tumor was identified on a hematoxylin and eosin (HE)-stained slide, and cores for the TMA were harvested from the corresponding tissue block using a Beecher Instruments Micro Tissue Arrayer. Eight cores of 0.6 mm were taken from the selected regions of the donor block of each tumor and inserted into recipient paraffin microarray blocks. Four μm thick sections of the fixed, paraffin embedded TMA tissue were cut with a microtome and placed on Superfrost slides. HE-staining and immunohistochemical cytokeratin-staining was performed to verify the presence of tumor tissue.
We experienced a loss of 16.5 % of the cores during preparation, and of the preserved cores, 8.4 % contained too few tumor cells to be evaluated. This loss due to technical issues is moderate compared to other reports [30, 31]. From each patient a mean number of 3.82 cores stained and evaluated for plectin expression.
Evaluated from each patient was 3.82.
Immunohistochemistry (IHC)
All slides were deparaffinised and rehydrated. The plectin antigen retrieval was enhanced by boiling in a pressurized system with 10 mM citrate buffer (pH 7.0). Further, the slides were incubated 30 min with 3 % H2O2 to block endogenous peroxidase activity, and incubated one hour with 10 % goat serum (Dako, Glostrup, Denmark) in phosphate buffered saline (PBS) (Dako, Glostrup, Denmark) to reduce unspecific staining. Rabbit primary monoclonal antibody against plectin (ab32528, Abcam, Cambridge, MA) was diluted 1:200 in Dako antibody diluent (S3022, Dako, Glostrup, Denmark), and incubated overnight at 4 °C. Subsequently, bound antibodies were visualized using the anti-rabbit Envision Plus System (K4011, Dako, Glostrup, Denmark). The slides were washed in PBS w/0.1 % Tween-20 with high concentrations of salt (0.4 M NaCl) and pH 6.0 (PBSTS6) after incubation with primary and secondary antibodies.
The anti-plectin antibody (ab32528) has previously been described and validated as a good marker for pancreatic adenocarcinoma [18]. As a negative control, pancreatic carcinoma tissue was treated according to the same staining protocol, but the primary antibody was omitted. No staining was seen indicating that the secondary antibody gave no unspecific staining in the tissue (data not shown).
Staining of normal oral mucosa showed specific and strong staining of muscle tissue in blood vessels, serving as positive control.
The uPAR staining was performed according to previous describes protocols [23].
Immunohistochemical scoring
All cores were examined by an experienced pathologist (SES) and a trained head and neck surgeon (OR) without knowledge of clinical outcome. The scoring was semi-quantitative, [32, 33] and the staining index (SI) calculated as a product of staining intensity (none (0), weak (1), moderate (2) or strong (3)) and proportion of positive tumor cells (no staining (0), <10 % (1), 10–50 % (2), 51–80 % (3) or >80 % (4)). Thus, the SI for each core differed from a minimum value of zero to a maximum of 12, and the patient’s final score was the mean SI of all cores evaluated. Tumors with score above the median value were classified as high expressers’, while those with score under median value were classified as having a low expression of the biomarker.
Immunofluorescence (IF)
For IF analysis the slides were pretreated as described in the IHC protocol for plectin. A rabbit polyclonal anti-plectin antibody (ab83497, Abcam, Cambridge, MA) recommended for IF was used. The antibody was diluted 1:10 in Dako antibody diluent (S3022, Dako, Glostrup, Denmark) and incubated for 3 h at room temperature. After the slides had been washed with PBSTS6, they were incubated overnight at 4 °C with the mouse monoclonal anti-human uPAR antibody (#3936, Sekisui Diagnostica, Stamford, CT, USA) at a 1:10 dilution in a buffer of 10 % goat serum (Dako North America, Carpintera, CA, USA) in PBS with 1 % BSA and 0.3 % Tween-20, pH 6.0. After incubation the slides were washed with PBSTS6. The secondary antibodies (Alexa Fluor 488, goat-anti rabbit, A11034 and Alexa Fluor 647, goat-anti mouse, A21236, Invitrogen, Carlsbad, CA) were mixed and diluted 1:200 in PBS. The slides were mounted and counterstained with DAPI antifade (DAPI in Fluorgard, 0.5 g/ml, Insitus, Albuquerque, NM) and sealed with nail polish. The slides were observed and photographed using Leica TCS SP5 confocal laser microscope with Leica Application Suite Advanced Fluorescence software (Leica Microsystems AG, Wetzlar, Germany).
The specificity of the polyclonal anti-plectin antibody (ab83497) used in the immunofluorescence staining was tested by staining pancreatic adenocarcinoma. Similar to the monoclonal anti-plectin antibody, the polyclonal anti-plectin antibody specifically stained pancreatic cancer cells. Furthermore, Western blotting of whole muscle cell lysate showed that the antibody gave a band of the approximately 500 kDa corresponding to the size of plectin (data not shown).
Statistics
All data were tabulated and analyzed using the IMB SPSS Statistics (Chicago, IL), version 21. Associations between different categorical variables were assessed by the Pearson`s chi-square test. Univariate analyses of the different variables’ influence on time to disease specific survival were performed using the Kaplan-Meier method, and statistical significance between categories was estimated by the log-rank test. Statistically significant values from COX univariate analyses were entered into a multivariate analysis using the backward stepwise Cox regression model. Disease specific death (DSD) was defined as patients dying form OSCC and not from unrelated conditions. These data were obtained from the “Cause of Death registry in Norway. Correlation between bivariates was calculated with Spearman’s rho. The cut-off for low and high expression for each biomarker was set at the median value of the patient’s final score, and was 5.60 for plectin and 5.63 for uPAR. All results were considered significant if p < 0.05, and reported according to the REMARK guidelines by McShane et al. [34] The starting point was defined as time of diagnosis, and the last day of follow up was January 1st, 2012.
Results
In the present study the prognostic value of plectin in OSCC was evaluated by a TMA-based immunohistochemical analysis of primary tumor tissue obtained from a North Norwegian cohort of 115 patients. The cohort consisted of 64 men and 51 women, with a mean age of 65.2 years (men 64.4/women 66.0) at diagnosis [23]. The majority of the cases presented with small tumors (T1, 34 % or T2, 37 %), no lymph node metastasis (N0, 63 %) and a well or moderately differentiated tumor (90 %). As expected, tumor size (T) and the presence of lymph node metastasis (N+) correlated significantly with disease specific death (DSD), while the grade of differentiation did not.
Immunohistological staining pattern
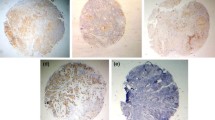
Plectin scarcely stained normal tongue mucosa that served as control material, while the blood vessels in the underlying stroma displayed stronger staining (Fig. 1a). In plectin-positive OSSC tumors the staining was relatively homogeneous throughout the tumor (Fig. 1b). The cancer cells were highly positive while the adjacent extracellular matrix showed less reactivity. The staining of the cancer cells was mainly confined to the plasma membrane (Fig. 2), but cytoplasmic staining was also observed in a relatively large proportion of the cores.
Immunohistochemical staining for plectin of normal tongue mucosa (a) and oral squamous cell carcinoma (b). The normal mucosa showed only faint staining while the blood vessels in the stroma were strongly stained. The staining of plectin positive tumor cells was strong, and the stroma showed almost no staining
Survival
For patients without lymph node metastasis (N0), at any T-stage, the risk of dying of the disease within 5 years was significantly decreased in patients with low plectin expressing tumors compared to those with high plectin expression (p = 0.008) (Table 1 and Fig 3). Also, for the patients with T1-tumors, independent of N-status, the statistical analyses revealed a significantly reduced risk for DSD in those who had tumors with low plectin expression (p = 0.031). As expected, given the preceding results, patients with T1N0 tumors with low plectin expression had an excellent outcome as all these patients were still alive after 5 years (p < 0.001). In a multivariate analysis of the N0-cases including plectin and tumor size (T1 vs T2-4), only high plectin expression was a significant independent predictor of increased DSD (p = 0.014, HR 3.674, CI 95 % 1.305-10.344).
Correlation of plectin and uPAR expression
We have recently shown that patients with T1N0 tumors expressing low levels of uPAR have a significantly reduced DSD compared to those with a higher uPAR expression. To investigate the distribution of expression of uPAR and plectin in the tumors we did a chi-square test, and found that a significant number of the tumors that were high expressers of uPAR also were high expressers of plectin, and those with low expression of uPAR generally were low expressers of plectin. This correlation was significant with a correlation coefficient of 0.769 (p = 0.01) as shown in a scatter plot in Fig. 4. Double immunofluorescence staining was performed on some selected tumors to investigate whether plectin and uPAR were co-located within the same cells, co-expressed by the same cells, or located to the same tumor regions. The immunofluorescence showed that the plectin and uPAR staining was localized to the same areas of the tumor (Fig. 5). As expected, the immunofluorescence staining confirmed that plectin was found mainly at the plasma membrane while uPAR was mainly localized in the cytoplasm.
Immunofluorescence staining of oral squamous cell carcinoma tissue. The majority of the cells were positive for both plectin and uPAR. Plectin (green) is located mainly at the plasma membrane and the periphery of the cell, while uPAR (red) is more prominent in the cytoplasmic part of the cells. Plectin is highly expressed in the wall of blood vessels (asterix)
Discussion
Surgery is the primary treatment for cancer in the oral cavity, and is often combined with radiotherapy while chemotherapy is more often applied as palliative treatment in late phase of the treatment [35–37]. Side effects of the treatment for oral cancer may be devastating for the patient’s quality of life, [38], and therefore it is important to avoid harmful overtreatment with radiotherapy of patients with small tumors with favorable prognosis. Decisions concerning the extent of treatment of patients with early stage OSCC are often difficult. Still, the choice of treatment is mainly based on TNM-stage which does not discriminate between aggressive and more indolent tumors. Several studies on potential biomarkers for treatment stratification have shown promising results, but so far no such biomarkers have been implemented in clinical practice. In the present study we have investigated the potential role of plectin as a prognostic biomarker. To our knowledge, the prognostic value of plectin in OSCCs has previously only been studied by Katada et al. who investigated a cohort of 62 HNSCC, among them 23 from the oral cavity [22]. Although the cohort was small and heterogeneous, they found that the survival was significantly decreased when the tumor cells expressed high levels of plectin. Our cohort is larger and composed only of tumors from the oral cavity and therefore more homogeneous. In accordance with the results from Katada et al., we found that low expression of plectin in the tumor cells predicts a favorable outcome, but only in patients with early stage disease.
We have previously found that low expression of uPAR correlated with reduced DSD for OSCC patients with T1N0 tumors, and that uPAR therefore might be a suitable prognostic marker for this subgroup of patients [23]. In the present study we found a highly significant correlation between the expression of plectin and uPAR. However, in contrast to uPAR, high expression of plectin correlated significantly with DSD in all patients with non-metastatic disease, and not only the T1N0 subgroup.
Most of the tumor cells that expressed plectin also expressed uPAR. It has previously been suggested that increased expression of uPAR in tumor cells is associated with EMT [27, 29, 39]. Little is known about plectin and EMT, but the formation of plectin-containing podosomes has been proposed as a first step towards EMT in OSCC [40].
Conclusion
The present study has shown that low expression of plectin predict a favorable outcome for patients with non-metastatic disease. Furthermore, all patients with T1N0 disease and low expression of plectin, survived for more than 5 years. Although the results must be validated in larger cohorts, they suggest plectin as a promising prognostic biomarker, which may be used to guide the treatment stratification for patients with non-metastatic OSCC.
Abbreviations
- DSD:
-
Disease specific death
- EMT:
-
Epithelial-mesenchymal transition.
- HNSCC:
-
Head and neck squamous carcinoma
- OSCC:
-
Oral squamous cell carcinoma
- TMA:
-
Tissue microarray
- TNM:
-
Classification system to evaluate the extent of primary tumor regional lymph node metastasis and distant metastasis
- uPAR:
-
urokinase plasminogen activator receptor
References
Barnes L, Eveson JW, Reichart P, Sidransky D (Eds): World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. IARC Press: Lyon 2005.
Franceschi S, Bidoli E, Herrero R, Munoz N. Comparison of cancers of the oral cavity and pharynx worldwide: etiological clues. Oral Oncol. 2000;36(1):106–15.
Cancer survival: England and Wales, less common cancers by age group. In. London: Office of National Statistics (ONS). Available at: http://www.cancerresearchuk.org/healthprofessional/cancer-statistics/statistics-by-cancer-type/oral-cancer/mortality#heading-Zero. Accessed January 2014.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer JClin. 2010;60(5):277–300.
Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;2:37.
Sobin LH, Gospodarowicz MK, Wittekind Ch (Eds): UICC International Union Against Cancer. TNM Classification of Malignant Tumours. 7th ed. Wiley-Blackwell 2009.
Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22.
Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):67–76.
Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5(4):311–6.
Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–78.
Brandwein-Gensler M, Smith RV, Wang B, Penner C, Theilken A, Broughel D, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34(5):676–88.
Brandwein-Gensler M, Wei S. Envisioning the next WHO head and neck classification. Head Neck Pathol. 2014;8(1):1–15.
Rodrigues PC, Miguel MC, Bagordakis E, Fonseca FP, de Aquino SN, Santos-Silva AR, et al. Clinicopathological prognostic factors of oral tongue squamous cell carcinoma: a retrospective study of 202 cases. Int J Oral Maxillofac Surg. 2014;43(7):795–801.
Soland TM, Brusevold IJ. Prognostic molecular markers in cancer - quo vadis? Histopathology. 2013;63(3):297–308.
Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: means, markers and perspectives (I). Oral Oncol. 2010;46(9):630–5.
Takes RP, Baatenburg De Jong RJ, Alles MJ, Meeuwis CA, Marres HA, Knegt PP, et al. Markers for nodal metastasis in head and neck squamous cell cancer. Arch Otolaryngol Head Neck Surg. 2002;128(5):512–8.
Wiche G, Krepler R, Artlieb U, Pytela R, Denk H. Occurrence and immunolocalization of plectin in tissues. J Cell Biol. 1983;97(3):887–901.
Bausch D, Thomas S, Mino-Kenudson M, Fernandez-del CC, Bauer TW, Williams M, et al. Plectin-1 as a novel biomarker for pancreatic cancer. Clin Cancer Res. 2011;17(2):302–9.
Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111(Pt 17):2477–86.
Gache Y, Chavanas S, Lacour JP, Wiche G, Owaribe K, Meneguzzi G, et al. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J Clin Invest. 1996;97(10):2289–98.
Osmanagic-Myers S, Gregor M, Walko G, Burgstaller G, Reipert S, Wiche G. Plectin-controlled keratin cytoarchitecture affects MAP kinases involved in cellular stress response and migration. J Cell Biol. 2006;174(4):557–68.
Katada K, Tomonaga T, Satoh M, Matsushita K, Tonoike Y, Kodera Y, et al. Plectin promotes migration and invasion of cancer cells and is a novel prognostic marker for head and neck squamous cell carcinoma. J Proteomics. 2012;75(6):1803–15.
Magnussen S, Rikardsen OG, Hadler-Olsen E, Uhlin-Hansen L, Steigen SE, Svineng G. Urokinase Plasminogen Activator Receptor (uPAR) and Plasminogen Activator Inhibitor-1 (PAI-1) Are Potential Predictive Biomarkers in Early Stage Oral Squamous Cell Carcinomas (OSCC). PLoS One. 2014;9(7), e101895.
Svineng G, Magnussen S, Hadler-Olsen E. Plasmino and the plasminogen activator system in health and disease. In: Extracellular Matrix Pathobiology and Signalling. Germany: Walter de Gruyter GmbH & Co; 2012. p. 261–90.
Blasi F, Sidenius N. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010;584(9):1923–30.
Ulisse S, Baldini E, Sorrenti S, D'Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr Cancer Drug Targets. 2009;9(1):32–71.
Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178(3):425–36.
Jo M, Lester RD, Montel V, Eastman B, Takimoto S, Gonias SL. Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem. 2009;284(34):22825–33.
Tang CH, Hill ML, Brumwell AN, Chapman HA, Wei Y. Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins. J Cell Sci. 2008;121(Pt 22):3747–56.
Chen B, van den Brekel MW, Buschers W, Balm AJ, van Velthuysen ML. Validation of tissue array technology in head and neck squamous cell carcinoma. Head Neck. 2003;25(11):922–30.
Mucci NR, Akdas G, Manely S, Rubin MA. Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Hum Pathol. 2000;31(4):406–14.
Kobel M, Weichert W, Cruwell K, Schmitt WD, Lautenschlager C, Hauptmann S. Epithelial hyaluronic acid and CD44v6 are mutually involved in invasion of colorectal adenocarcinomas and linked to patient prognosis. Virchows Arch. 2004;445(5):456–64.
Metindir J, Dilek GB, Pak I. Staining characterization by immunohistochemistry of tumor cancer antigen in patients with endometrial cancer. Eur J Gynaecol Oncol. 2008;29(5):489–92.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(36):9067–72.
Franceschi D, Gupta R, Spiro RH, Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg. 1993;166(4):360–5.
Brown JS, Rogers SN, Lowe D. A comparison of tongue and soft palate squamous cell carcinoma treated by primary surgery in terms of survival and quality of life outcomes. Int J Oral Maxillofac Surg. 2006;35(3):208–14.
Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, Bentzen J,Bastholt L, Hansen O, Johansen J, Andersen L, Evensen JF. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362(9388):933-40.
Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. Complications of radiation therapy for head and neck cancers. The patient's perspective. Cancer Nurs. 2002;25(6):461–7. quiz 468–469.
Huang C, Xie D, Cui J, Li Q, Gao Y, Xie K. FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal transition and metastasis via upregulation of expression of the urokinase plasminogen activator system. Clin Cancer Res. 2014;20(6):1477–88.
Takkunen M, Hukkanen M, Liljestrom M, Grenman R, Virtanen I. Podosome-like structures of non-invasive carcinoma cells are replaced in epithelial-mesenchymal transition by actin comet-embedded invadopodia. J Cell Mol Med. 2010;14(6B):1569–93.
Acknowledgements
We thank Bente Mortensen, Eli Berg and Magnus Persson for excellent technical assistance, and Inger Sperstad for help with designing the database. In addition we thank Bodil Fadnes for assistance with immunofluorescence staining and technical issues. This work was supported The North Norwegian Regional Health Authorities and UiT The Artic University of Norway, both being non-profit organizations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
OGR collected the material, carried out immunohistochemical and immunofluorescence staining, interpreted data, performed statistical analysis, and drafted the manuscript. SNM carried out immunohistochemical staining, interpreted data and drafted the manuscript. Gs and EHO participated in the design of the study, contributed to interpretation of data, and critically revised the manuscript. LUH conceived the design of the study, contributed to interpretation of data, and critically revised the manuscript. SES participated in the design of the study, contributed to interpretation of data, performed statistical analysis, and revised the manuscript critically. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rikardsen, O.G., Magnussen, S.N., Svineng, G. et al. Plectin as a prognostic marker in non-metastatic oral squamous cell carcinoma. BMC Oral Health 15, 98 (2015). https://doi.org/10.1186/s12903-015-0084-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-015-0084-9