Abstract
Background
Type 1 diabetes mellitus (T1DM) is well-known to trigger a disruption of lipid metabolism. This study aimed to compare lipid profile changes in T1DM patients after achieving glucose control and explore the underlying mechanisms. In addition, we seek to identify novel lipid biomarkers associated with T1DM under conditions of glycemic control.
Methods
A total of 27 adults with T1DM (age: 34.3 ± 11.2 yrs) who had maintained glucose control for over a year, and 24 healthy controls (age: 35.1 + 5.56 yrs) were recruited. Clinical characteristics of all participants were analyzed and plasma samples were collected for untargeted lipidomic analysis using mass spectrometry.
Results
We identified 594 lipid species from 13 major classes. Differential analysis of plasma lipid profiles revealed a general decline in lipid levels in T1DM patients with controlled glycemic levels, including a notable decrease in triglycerides (TAGs) and diglycerides (DAGs). Moreover, these T1DM patients exhibited lower levels of six phosphatidylcholines (PCs) and three phosphatidylethanolamines (PEs). Random forest analysis determined DAG(14:0/20:0) and PC(18:0/20:3) to be the most prominent plasma markers of T1DM under glycemic control (AUC = 0.966).
Conclusions
The levels of all metabolites from the 13 lipid classes were changed in T1DM patients under glycemic control, with TAGs, DAGs, PCs, PEs, and FFAs demonstrating the most significant decrease. This research identified DAG(14:0/20:0) and PC(18:0/20:3) as effective plasma biomarkers in T1DM patients with controled glycemic levels.
Similar content being viewed by others
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disorder characterized by the destruction of islet β cells, resulting in hyperglycemia due to impaired insulin secretion [1]. It has an estimated incidence of 15 cases per 100,000 and a prevalence rate of 9.5%, both showing an increasing trend globally [2]. With multifactorial and polygenic etiology, T1DM demonstrates high heterogeneity across individuals [3,4,5]. While T1DM is commonly associated with childhood and adolescence [6], it can manifest at any age. Recent data from the UK Biobank reveal that up to 42% of T1DM cases are diagnosed in individuals aged between 30 and 60 years [7]. At present, insulin injection can effectively control the blood glucose levels of patients with T1DM [8]. However, hyperglycemia can lead to a series of metabolic disorders, with lipid metabolic disorder being a significant manifestation.
Lipid metabolism involves a complex interplay of various lipid species that are crucial for maintaining cellular structure, energy storage, and signaling processes [9]. Metabolic abnormalities in lipids and amino acids are common in children who develop symptomatic T1DM [10, 11]. Higher levels of triglycerides (TAGs) and apolipoprotein B (apoB) are found correlated with elevated risk of T1DM [10, 12]. TAGs and other lipids manifest markedly elevated levels in children with positive autoantibody [12]. These suggest that blood lipid levels may serve as predictors for T1DM diagnosis. In T1DM, dysregulation of lipid metabolism manifests as altered levels of lipid classes, including TAGs, diglycerides (DAGs), phosphatidylcholines (PCs), and phosphatidylethanolamines (PEs) [13, 14]. These changes can provide insights into the metabolic adaptations and potential vulnerabilities in T1DM patients.
Traditionally, blood autoantibodies against cellular antigens, such as insulin, glutamic acid decarboxylase antibodies (GADAs), islet antigen 2 (IA-2), and zinc transporters are employed for T1DM prediction [15]. Moreover, gene expression analysis, metabolomics, proteomics, and epigenetics may provide evidence and biomarkers to predict and diagnose T1DM [11, 16, 17]. Recent advancements in mass spectrometry and bioinformatics have facilitated profound improvements in determining and interpreting lipidomics data [18, 19]. Current lipidomic studies have significantly broadened the profiling of lipids previously less well-known. A population-based cohort study detected 14 sphingolipids as significantly associated with elevated risk of T2DM [20], which confirmed the positive associations of SM C34:1 and SM C36:1with T2DM in a Singapore Chinese population [21], as well as of Cer(d18:1/20:0) with T2DM in a French population [22]. However, despite these advancements, comprehensive plasma lipidomics analysis of T1DM remains limited. Understanding the lipidome alterations of T1DM could help unveil the fingerprints associated with dyslipidemia and cardiovascular diseases, which may serve as a valuable screening tool for T1DM.
Despite advancements in glycemic management strategies, achieving optimal glycemic control remains challenging for many T1DM patients. Moreover, the impact of glycemic control on lipid metabolism in these patients is not well understood. Previous studies have primarily focused on the immediate effects of hyperglycemia on lipid profiles, with insufficient attention given to long-term lipid changes after sustained glycemic control [23, 24]. This study aimed to address this gap by investigating the lipidomic alterations in T1DM patients who have achived glycemic control. Using untargeted lipidomic analysis, we compared the lipid profiles of these T1DM patients to those of healthy participants, aiming to identify significant lipid changes and potential biomarkers. Understanding these lipidomic changes is crucial for developing better diagnostic tools and therapeutic strategies to manage glycemic control in T1DM.
Materials and methods
Clinical samples and baseline data
A total of 27 T1DM patients under glycemic control (T1DMGC) were recruited in the Department of Endocrinology, the First People’s Hospital of Yunnan Province from January 2020 to January 2021. Inclusion criteria were as follows: 1) Patients aged between 18–45 years at the time of recruitment; 2) Patients diagnosed with T1DM for more than one year and received multiple-dose insulin injections (MDII); 3) The level of glycated hemoglobin (HbA1c) was less than 9% (75 mmol/mol). Patients with abnormal thyroid function and ketoacidosis within one month before recruitment were excluded. Meanwhile, 24 age-matched healthy adults were selected in the physical examination center of the same hospital as the control group during the same period. Clinical and biochemical features of the study population are presented in Table 1. Detailed information of BMI are also listed in Supplementary Table 1.
A written informed consent was acquired from all participants before inclusion. The study was approtableved by the Medical Ethics Committee of the First People’s Hospital of Yunnan Province (No. KHLL2016-KY038). All datasets were anonymized, with blood samples collected and stored following REMARK for biomarker analysis.
Lipidomic analyses
Total lipids from plasma samples were analyzed using a modified Folch procedure [25]. Plasma Lipids were extracted from 100 μL plasma using a chloroform: methanol (2:1 v/v) solvent system after internal standards were added (stable isotope labeled & non-physiological lipid species). Lipids were quantified by a high-throughput quantitative lipidomics method [26]. The following lipid standards were used: 1,2-dimyristoyl-sn-glycero-3-phospho(choline-d13)(PC(14:0)-d13)), 1,2,3-triheptadecanoylglycerol (TG(17:0/17:0/17:0)), 3β-hydroxy-5-cholestene 3-linoleate (ChoE(18:2)), 1,2-diheptadecanoyl-sn-glycero-3-phosphoethanolamine (PE(17:0/17:0)), N-heptadecanoyl-D-erythro-sphingosylphosphorylcholine (SM(d18:1/17:0)), N-heptadecanoyl-D-erythro-sphingosine (Cer(1/17:0)-d18), 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine (PC(17:0/17:0)), 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC(17:0)), 1-pal -mitoyl-d31-2-oleoyl-sn-glycero-3-phosphocholine (PC(16:0/d31/18:1)), 1-hexad -ecyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine (PC(16:0e/18:1(9Z))), 1-(1Z-octadecenyl)-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine (PC(18:0p/18:1(9Z))), 1-octadecanoyl-sn-glycero-3-phosphocholine (LPC(18:0)), 1-(1Z-octadecenyl)-2-docosahexaenoyl-sn-glycero-3-phosphocholine (PC(18:0p/22:6)) from Sigma-Aldrich Inc; 1-stearoyl-2-linoleoyl-sn-glycerol (DG(18:0/20:4)) from Avanti Polar Lipids Inc (Alabaster, AL, USA) and tripalmitin-1,1,1-13C3 (TG(16:0/16:0/16:0)-13C3), trioctanoin-1,1,1-13C3 (TG(8:0/8:0/8:0)-13C3) and 1-palmitoyl-2-hydroxy-sn-glycero-3phosphatidylcholine (LPC(16:0)) from Larodan AB Inc (Solna, Sweden).
Ultra High-Performance Liquid Chromatography-MS/MS (UHPLC-MS/MS) analysis
UHPLC separation was achieved using a SCIEX ExionLC series UHPLC System (Sciex, Framingham, USA), equipped with ACQUITY UPLC HSS T3 column (1.8 μm 2.1*100 mm, Waters Corp., Milford, USA). The mobile phase A was 40% water, 60% acetonitrile, and 10 mmol/L ammonium formate, while the mobile phase B was 10% acetonitrile, 90% isopropanol, and 10 mmol/L ammonium formate. The column temperature was set at 40 ℃. The auto-sampler temperature was at 6 ℃, and the injection volume was 2 μL. Further, an AB Sciex QTrap 6500 + mass spectrometer was applied for assay development. The ion source parameters were: ion spray voltage: + 5500/-4500 V, curtain gas: 40 psi, temperature: 350℃, ion source gas: 1:50 psi, ion source gas 2: 50 psi, and DP: ± 80 V.
Data pre-processing
Mass spectrometric lipidomic data were preprocessed with MZmine 2.18.2 [27]. The workflow consisted of raw data importing, filtering, peak detection, chromatogram construction, chromatogram deconvolution, peak list de-isotoping, peak list alignment, gap filling, and peak annotation. This was achieved by integrating mass spectrometry and retention time information using an in-house lipid library with a m/z tolerance of 0.006 m/z and an RT tolerance of 0.2 min. Pre-processing and analysis of lipidomics data were conducted using R software (v. 4.3.1). Pre-processed data were normalized according to internal specifications. Using the k-nearest neighbor technique, missing values of the lipidomic data set were retrieved. Log transformation was applied for a normal distribution. The coefficient of variation (relative standard deviation; %RSD) for peak regions and retention durations of lipid-class-specific internal standards were calculated. Lipid classes were determined by summing up individual lipid species within each class.
Statistical processing
R was utilized for data analysis and visualization. Four repeated measurements from each participant were analyzed by a mixed-effect model for lipid species. All statistical tests were adjusted for multiple tests using the Benjamini and Hochberg procedure. Twenty-five tests were adjusted for multiple comparisons based on the false discovery rate (FDR). Initially, the differences between the two groups were modeled with a lipid species-wise mixed-effect model, with time-diet interaction as a fixed effect and participants as a random effect.
Results
Clinical studies and statistical information
Plasma samples were collected from the 27 T1DMGC patients (34.3 ± 11.2 yrs) and 24 healthy controls (35.1 ± 5.5 yrs). No significant variations existed in age, gender, and body weight (Table 1). However, biochemical tests demonstrated substantial differences between the two groups in several plasma parameters. Briefly, T1DMGC patients had significantly lower levels of low-density lipoproteins (LDLs) (p < 0.01) and TAGs (Fig. 1, p < 0.001). Furthermore, plasma levels of uric acids (UA) and alanine aminotransferase (ALT) decreased sharply (p < 0.05). In contrast, the levels of HbA1c (p < 0.001) and fasting blood glucose (FBG, p < 0.01) were elevated. Higher levels of HbA1c in patients with T1DM usually indicate higher levels of blood glucose. In T1DMGC, HbA1c is directly correlated with cholesterol. TAGs and LDLs are negatively correlated with high-density lipoproteins (HDLs). There was a linear relationship between HbA1c and dyslipidemia.
Clinical phenotypic differences in plasma samples between type 1 diabetes (DM) patients and healthy controls (CON). LDL: Low-density lipoprotein, TAG: triglyceride, UA: uric acid, HbA1c: glycosylated hemoglobin, FBG: fasting blood glucose, ALT: alanine aminotransferase. *** denoting statistical test with p < 0.001, ** statistical test with p < 0.01 and * statistical test with p < 0.05 according to Wilcoxon test
Untargeted lipidomics detected alterations in plasma lipids in T1DMGC
Through UHPLC-Q-TOF–MS, a total of 594 lipid species from 13 major lipid classes were identified, such as TAGs, diglycerides (DAGs), PCs, phosphatidylethanolamines (PEs), hexosylceramides (HexCers), lysophosphatidylethanolamines (LPEs), lysophosphatidylcholines (LPCs) and lactosylceramides (LCERs). TAG species dominated with 372 metabolites, followed by PCs, PEs, and DAGs, each having 40, 37, and 30 detectable metabolites, respectively. (Figure S1A). Partial least squares discriminant (PLS-DA) analysis revealed marked differences in lipid composition between the T1DM and normal groups (Figure S1B). Moreover, the levels of most lipid metabolites declined in T1DM patients, with the notable exceptions of LPCs and LPEs (Figure S1C). In T1DMGC patients, TAGs showed the greatest relative decrease, followed by DAGs (Figure S1D). In summary, plasma lipid composition of patients with T1DMGC was significantly changed from that of the healthy population.
Alterations in glycerides and phospholipids in T1DMGC
Differential analysis of all metabolites was conducted to examine the altering patterns of lipid molecules in T1DMGC. The results revealed that 224 TAGs (Fig. 2A) and 22 DAGs (Fig. 2B) were down-regulated in T1DMGC. Only one DAG was found elevated. TAGs constitute the primary component in vegetable and animal fats, serving as the principal component of adipose tissue stores. An extensive decrease of TAGs may indicate dyslipidemia in T1DMGC patients. These findings concurred with the previous results in this study that T1DMGC patients had low levels of LDLs. Compared with the control group, the levels of six PCs (Fig. 3A) and three PEs (Fig. 3B) were all reduced. Most lipid metabolites exhibited a declining trend in T1DMGC plasma samples, allowing us to seek molecular markers for clinical diagnosis.
Interconversion of plasma lipids in patients with T1DMGC
Alterations in overall lipid composition in T1DMGC may be caused by the mutual transformation of lipids.The levels of TAGs in T1DMGC were lower than those in the control group (Fig. 4). TAGs are synthesized in vivo in two ways: one is the esterification pathways, in which fatty acids are esterified with glycerol by monoacylglycerol acyltransferase (MGAT) and diacylglycerol acyltransferase (DGAT); the other is the ab initio synthesis, which starts with 3-phosphoglycerate (G3P) and free fatty acids. Our results showed that esterification is the main way to synthesize TAGs in T1DMGC (Fig. 4).
There was no significant difference in the levels of PEs and PCs between the T1DMGC and the healthy group. Glycerol phospholipids contain at least one acyl, alkyl or alkenyl-linked fatty acyl side chain, which can be divided into phosphatidic acids (PAs), phosphatidylglycerols (PGs), phosphatidylinositols (PIs), PCs, PEs, phosphatidylserine (PS) and lysophosphatidylphospholipids (lyso-PL) after hydrolyzing a fatty acid side chain. The metabolism and mutual transformation of phospholipids form a complex network, which is regulated by a variety of metabolic enzymes. PCs and PEs are the most abundant phospholipids, which were dynamically balanced in T1DMGC. PEs can produce PCs through the PEMT pathway, which becomes another critical pathway for the source of PCs.
Changes in blood lipids can also be manifested by the level of sphingomyelin. The common structure of sphingomyelins is a sphingosine skeleton and a long-chain fatty acid side chain, mainly synthesized from ab initio in the endoplasmic reticulum. SM and ceramide (Cer) can be transformed into each other by sphingomyelin synthase (SGMS) and sphingomyelin hydrolase (SMPD). In addition to the hydrolysis of SM, ceramide can also be synthesized from scratch by serine and palmitoyl coenzyme A or by ceramide synthase (CERS).T1DMGC patients displayed no significant change in the level of SM but had decreased level of Cer compared with the control group (Fig. 4).
Identifying effective indicators of T1DMGC
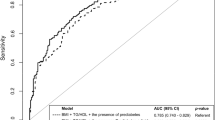
Finally, we tried to detect biomarkers with high predictive potential in T1DMGC patients. Random forest was adopted to estimate individual classification capabilities using AUC (area under the curve). The top 20 lipid metabolites with the highest classification accuracy were selected (Fig. 5A), with DAG(14:0/20:0)(AUC = 0.966), DAG(16:0/16:0) (AUC = 0.941), DAG(16:0/18:0)(AUC = 0.934), PC(18:0/20:3)(AUC = 0.92) and TAG(56:7)FA18:1(AUC = 0.948) to be the five biomarkers with the highest classification capability (Fig. 5B). In conclusion, the above five biomarkers may serve as highly accurate and novel indicators for clinical detection, diagnosis, and detection of T1DMGC.
Discussion
Our study identified significant changes in the plasma levels of TAGs, DAGs, PCs, and PEs in T1DM patients with controlled glycemic levels, suggesting a complex interplay between lipid species and glycemic-controlled T1DM. Abnormal levels of specific lipid species may contribute to the dysregulation of glucose homeostasis, thereby promoting the onset and progression of T1DM [28, 29]. The observed alterations in lipid profiles between T1DMGC and healthy groups provided valuable insights into the impact of glycemic control on lipid metabolism in T1DM.
Previous studies have established the higher prevalence of dyslipidemia in individuals with T1DM compared with non-diabetic populations [30]. Acute hyperglycemia has been shown to significantly alter lipid metabolism, often resulting in elevated levels of TAGs, low- LDL cholesterol, along with a reduction in HDL cholesterol [31]. These alterations can exacerbate the risk of cardiovascular diseases. Glycemic control plays a critical role in modulating lipid abnormalities in T1DM. Patients with inadequate glycemic control showed markedly elevated TAG levels and decreased high-density HDL cholesterol. As glycemic control deteriorated, alterations in LDL particle subclasses were noted, with a pronounced decrease in LDL levels among these individuals [32]. Conversely, T1DM patients who maintained good glycemic control exhibited lipid profiles closely resembling those of the general population [33]. Interestingly, a notable lack of literature addresses non-targeted lipid profiling in T1DM. Our research presents novel findings, revealing significant differences in TAGs, DAGs, PCs, and PEs in T1DM patients with HbA1c levels below 9%, compared to normal controls. This innovative approach highlights the necessity for further exploration of lipid profiles in T1DM with controlled glycemic levles to better understand its implications for cardiovascular risk.
Lipids are crucial macromolecules performing critical tasks in the body. The lipidome composition may be influenced by dietary lipid intake in T1DM patient [34, 35]. Our untargeted lipidomic analysis revealed significant changes across 13 major lipid classes in T1DM patient post-glucose control, with TAGs, DAGs, PCs, PEs, and FFAs exhibiting the most dramatic changes. Notably, TAGs and DAGs, which are key in energy storage and signaling, showed marked declines [36]. This decline might indicate a link between impaired TAG metabolism and disrupted blood glucose control, a key issue in T1DM management. Traditional clinical markers of diabetes include TAGs, cholesterol, and non-esterified fatty acids [37, 38]. However, these plasma lipids are only of limited value in identifying individuals at risk for developing diabetes and fail to determine which individuals may progress into diabetes. Here, highly effective plasma biomarkers were detected for assessing glycmic control status in T1DM, such as DAG(14:0/20:0) and PC(18:0/20:3).
Phospholipids such as PCs and sphingomyelins are two significant groups of phospholipids crucial for normal functions of cell membranes [39, 40]. Phospholipids are also a substantial component of HDL, particularly in variants containing polyunsaturated fatty acids. This study revealed that plasma samples from T1DMGC patients had considerably lower levels of PCs and PEs. The altered levels of PCs and sphingomyelins further emphasize the importance of membrane lipid composition in T1DMGC. These lipids play critical roles in cellular function, whose dysregulation may disrupt insulin signaling pathways, contributing to the hyperglycemic state observed in T1DM [41]. A lower plasma level of HDL cholesterol hinted at a positive association between levels of polyunsaturated sphingomyelin and PCs and the level of HDL cholesterol [42, 43].
As demonstrated by previous research, β-cell inflammation is modulated by aberrant sphingolipid levels during an early stage of T1DM [20, 44]. Sphingolipids are comprised of sphingomyelin (SM) and glycosphingolipid (GSP), both sharing a similar structural framework of a sphingosine backbone and a long fatty acid side chain. Sphingolipids are predominantly generated from scratch within the endoplasmic reticulum. Both sphingomyelin synthase (SGMS) and sphingomyelin hydrolase (SMH) can interconvert SM and ceramide. Furthermore, SM can be decomposed into ceramide via hydrolysis, which can also be synthesized de novo using serine and palmitoyl-CoA or by ceramide synthase (CERS). Both ceramide and dihydroceramide (DCERs) were significantly down-regulated in T1DMGC patients. We thus postulated that this might be a root cause of aberrant inflammatory response. Autoimmune response against pancreatic beta cells is probably a significant causative factor in T1DM. By activating critical signaling lipids such as lysin lecithin, prostaglandins, and other arachidonic acid derivatives and certain sphingolipids, inflammation has been directly correlated with multiple sclerosis and rheumatoid arthritis [45, 46]. Therefore, the aberrant levels of sphingolipids in plasma samples of T1DM patients may implicate inappropriate immunological responses. One notable finding is the decrease in LDL levels in T1DMGC patients, which may be attributed to the overall metabolic alterations and the effects of glycemic control measures [47]. The reduction in LDL cholesterol could be a compensatory mechanism or a result of altered lipid metabolism pathways in T1DMGC [48]. ALT is a marker of liver function and has been associated with metabolic disorders. Lower ALT levels in T1DMGC patients might indicate reduced hepatic lipid synthesis or altered liver function due to chronic hyperglycemia and its management [49]. Monitoring ALT levels could provide insights into the hepatic involvement in T1DMGC and help in managing the disease more effectively.
Our study unveils crucial insights into the lipidomic alterations associated with T1DM, offering potential clinical insights in several key areas. The distinct lipid profiles observed in T1DM individuals post-glucose control may pave the way for developing a lipidomic signature that can aid in assessing glycemic control status in T1DM and differentiating it from other metabolic disorders. While this study provides valuable insights, it is essential to acknowledge certain limitations that may impact the generalizability of our findings. Firstly, the cross-sectional nature of the study design limits our ability for analyses of causation. Longitudinal studies are warranted to elucidate the temporal relationships between lipid alterations and T1DM management and progression. Furthermore, the sample size of the study may decrease the generalizability of obtained results. Larger, more diverse cohorts are necessary to account for potential variations in lipidomic profiles among different demographic groups.
Conclusions
This study presents novel insights into the lipidomic alterations in T1DMGC patients after achieving glucose control, a relatively underexplored area in diabetes research. While previous studies have primarily focused on the immediate effects of hyperglycemia on lipid profiles, our research delves into the long-term lipid changes under glycemic control, highlighting the significance of sustained metabolic management. Our findings reveal substantial changes in plasma levels of various lipid species, particularly TAGs, DAGs, PCs, and PEs. The identification of specific lipid biomarkers such as DAG(14:0/20:0) and PC(18:0/20:3) offers new tools for assessing glycemice control status in T1DM, potentially enabling better differentiation from other metabolic disorders. The significance of our study lies in its contribution to the understanding of lipid metabolism in T1DM, particularly after glycemic control, which has been largely overlooked in existing literature. By elucidating the lipid profile alterations, we provide a deeper understanding of the metabolic disturbances in T1DM, emphasizing the complex interplay between lipid species and glucose homeostasis. Future research should focus on longitudinal studies to further explore the temporal relationships between lipid alterations and T1DM management. Larger, more diverse cohorts are also necessary to validate our findings and ensure their generalizability across different populations. Additionally, investigating the mechanisms underlying the observed lipid changes can uncover new therapeutic targets, enhancing the management of T1DM. In summary, our study underscores the importance of lipidomics in understanding glycemic control in T1DM and in differentiating it from other metabolic disorders. These findings may ultimately contribute to more effective management strategies and better patient outcomes.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the complexity and amount of data, which requires special processing software. However, data are available from the corresponding author on reasonable request.
Abbreviations
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- TAGs:
-
Triglycerides (TAGs)
- apoB:
-
Apolipoprotein B
- GADAs:
-
Glutamic acid decarboxylase antibodies
- UHPLC-MS/MS:
-
Ultra High-Performance Liquid Chromatography-MS/MS
- FDR:
-
False discovery rate
- ALT:
-
Uric acids (UA) and alanine aminotransferase
- FBG:
-
Fasting blood glucose
- HDLs:
-
High-density lipoproteins
- DAGs:
-
Diglycerides
- Pes:
-
Phosphatidylethanolamines
- HexCers:
-
Hexosylceramides
- LPEs:
-
Lysophosphatidylethanolamines
- LPCs:
-
Lysophosphatidylcholines
- LCERs:
-
Lactosylceramides
- PCs:
-
Phosphatidylcholines
- PLS-DA:
-
Partial least squares discriminant
- Pas:
-
Phosphatidic acids
- PGs:
-
Phosphatidylglycerols
- PIs:
-
Phosphatidylinositols
- PS:
-
Phosphatidylserine
- CERS:
-
Ceramide synthase
- SGMS:
-
Sphingomyelin synthase
- SMPD:
-
Sphingomyelin hydrolase
- SGMS:
-
Sphingomyelin synthase
- SMH:
-
Sphingomyelin hydrolase
References
Brenu EW, Harris M, Hamilton-Williams EE. Circulating biomarkers during progression to type 1 diabetes: a systematic review. Front Endocrinol. 2023;14: 1117076.
Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Fard HH, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Health Promot Perspect. 2020;10(2):98.
Association AD. Management of dyslipidemia in children and adolescents with diabetes. Diabetes Care. 2003;26(7):2194–7.
Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2009;10:3–12.
Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, Holl RW. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care. 2007;30(10):2523–8.
Wadwa RP, Reed ZW, Buckingham BA, DeBoer MD, Ekhlaspour L, Forlenza GP, Schoelwer M, Lum J, Kollman C, Beck RW. Trial of hybrid closed-loop control in young children with type 1 diabetes. N Engl J Med. 2023;388(11):991–1001.
Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018;6(2):122–9.
Tatovic D, Narendran P, Dayan CM. A perspective on treating type 1 diabetes mellitus before insulin is needed. Nat Rev Endocrinol. 2023;19(6):361–70.
Petrenko V, Sinturel F, Riezman H, Dibner C. Lipid metabolism around the body clocks. Prog Lipid Res. 2023;91:101235.
Oresic M, Simell S, Sysi-Aho M, Nanto-Salonen K, Seppanen-Laakso T, Parikka V, Katajamaa M, Hekkala A, Mattila I, Keskinen P. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med. 2008;205(13):2975–84.
Pflueger M, Seppänen-Laakso T, Suortti T, Hyötyläinen T, Achenbach P, Bonifacio E, Orešič M, Ziegler AG. Age-and islet autoimmunity–associated differences in amino acid and lipid metabolites in children at risk for type 1 diabetes. Diabetes. 2011;60(11):2740–7.
Lamichhane S, Ahonen L, Dyrlund T. Dynamics of plasma lipidome in progression to islet autoimmunity and type 1 diabetes–type 1 diabetes prediction and prevention study (DIPP). Sci Rep. 2018;8(1):10635.
Julve J, Genua I, Quifer-Rada P, Yanes Ó, Barranco-Altirriba M, Hernández M, Junza A, Capellades J, Granado-Casas M, Alonso N. Circulating metabolomic and lipidomic changes in subjects with new-onset type 1 diabetes after optimization of glycemic control. Diabetes Res Clin Pract. 2023;197: 110578.
Parker A, Kim Y. The effect of low glycemic index and glycemic load diets on hepatic fat mass, insulin resistance, and blood lipid panels in individuals with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2019;17(8):389–96.
Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci. 2007;104(43):17040–5.
Kallionpää H, Elo LL, Laajala E, Mykkänen J, Ricaño-Ponce I, Vaarma M, Laajala TD, Hyöty H, Ilonen J, Veijola R. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes. 2014;63(7):2402–14.
Moulder R, Bhosale SD, Erkkilä T, Laajala E, Salmi J, Nguyen EV, Kallionpää H, Mykkänen J, Vähä-Mäkilä M, Hyöty H. Serum proteomes distinguish children developing type 1 diabetes in a cohort with HLA-conferred susceptibility. Diabetes. 2015;64(6):2265–78.
Wu Z, Shon JC, Liu KH. Mass spectrometry-based lipidomics and its application to biomedical research. J Lifestyle Med. 2014;4(1): 17.
Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003;44(6):1071–9.
Yun H, Sun L, Wu Q, Zong G, Qi Q, Li H, Zheng H, Zeng R, Liang L, Lin X. Associations among circulating sphingolipids, β-cell function, and risk of developing type 2 diabetes: a population-based cohort study in China. PLoS Med. 2020;17(12): e1003451.
Chew WS, Torta F, Ji S, Choi H, Begum H, Sim X, Khoo CM, Khoo EYH, Ong WY, Van Dam RM. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI insight. 2019;4(13):e126925.
Wigger L, Cruciani-Guglielmacci C, Nicolas A, Denom J, Fernandez N, Fumeron F, Marques-Vidal P, Ktorza A, Kramer W, Schulte A. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 2017;18(9):2269–79.
Kamrul-Hasan A, Talukder SK, Kabir MA, Mustari M, Un Nabi MM, Gaffar AJ, Hossain MF, Alam MS, Islam MR, Hannan MA. Comparison of fasting and random lipid profiles among subjects with type 2 diabetes mellitus: an outpatient-based cross-sectional study in Bangladesh. Diabetol Metab Syndr. 2023;15(1):139.
Ji L, Weng J, Lu J, Guo X, Yang W, Jia W, Zou D, Zhou Z, Zhu D, Ji Q. Hyperglycemia and duration of diabetes as risk factors for abnormal lipids: a cross sectional survey of 19,757 patients with type 2 diabetes in China. J Endocrinol Invest. 2014;37:843–52.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509.
Tu J, Yin Y, Xu M, Wang R, Zhu ZJ. Absolute quantitative lipidomics reveals lipidome-wide alterations in aging brain. Metabolomics. 2018;14:1–11.
Pluskal T, Castillo S, Villar-Briones A, Orešič M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010;11(1):1–11.
Meneses MJ, Silvestre R, Sousa-Lima I, Macedo MP. Paraoxonase-1 as a regulator of glucose and lipid homeostasis: impact on the onset and progression of metabolic disorders. Int J Mol Sci. 2019;20(16): 4049.
Sobczak AI, Pitt SJ, Smith TK, Ajjan RA, Stewart AJ. Lipidomic profiling of plasma free fatty acids in type-1 diabetes highlights specific changes in lipid metabolism. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(1):158823.
Suvitaival T. Lipidomic abnormalities during the pathogenesis of type 1 diabetes: a quantitative review. Curr Diabetes Rep. 2020;20:1-9 %@ 1534-4827.
Tricò D, Mengozzi A, Baldi S, Bizzotto R, Olaniru O, Toczyska K, Huang GC, Seghieri M, Frascerra S, Amiel SA. Lipid-induced glucose intolerance is driven by impaired glucose kinetics and insulin metabolism in healthy individuals. Metabolism. 2022;134: 155247.
Vergès B. Dyslipidemia in type 1 diabetes: a masked danger. Trends Endocrinol Metab. 2020;31(6):422–34.
Zhou Z, Sun B, Huang S, Zhu C, Bian M. Glycemic variability: adverse clinical outcomes and how to improve it? Cardiovasc Diabetol. 2020;19:1–14.
Ritchie RH, Zerenturk EJ, Prakoso D, Calkin AC. Lipid metabolism and its implications for type 1 diabetes-associated cardiomyopathy. J Mol Endocrinol. 2017;58(4):R225–40.
Vergès B. Lipid disorders in type 1 diabetes. Diabetes Metab. 2009;35(5):353–60.
Mäkinen VP, Soininen P, Kangas AJ, Forsblom C, Tolonen N, Thorn L, Viikari J, Raitakari O, Savolainen M, Groop PH. Triglyceride-cholesterol imbalance across lipoprotein subclasses predicts diabetic kidney disease and mortality in type 1 diabetes: the FinnDiane study. J Intern Med. 2013;273(4):383–95.
Lin J, Hu FB, Rimm EB, Rifai N, Curhan GC. The association of serum lipids and inflammatory biomarkers with renal function in men with type II diabetes mellitus. Kidney Int. 2006;69(2):336–42.
Sasongko MB, Wong TY, Nguyen TT, Kawasaki R, Jenkins A, Shaw J, Wang JJ. Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care. 2011;34(2):474–9.
Liebisch G, Lieser B, Rathenberg J, Drobnik W, Schmitz G. High-throughput quantification of phosphatidylcholine and sphingomyelin by electrospray ionization tandem mass spectrometry coupled with isotope correction algorithm. Biochim Biophys Acta Mol Cell Biol Lipids. 2004;1686(1–2):108–17.
Yen CLE, Mar MH, Zeisel SH. Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. FASEB J. 1999;13(1):135–42.
Meikle PJ, Summers SA. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017;13(2):79–91.
Yancey PG, de la Llera-Moya M, Swarnakar S, Monzo P, Klein SM, Connelly MA, Johnson WJ, Williams DL, Rothblat GH. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J Biol Chem. 2000;275(47):36596–604.
Zakiev E, Rached F, Lhomme M, Darabi-Amin M, Ponnaiah M, Becker PH, Therond P, Serrano CV Jr, Santos RD, Chapman MJ. Distinct phospholipid and sphingolipid species are linked to altered HDL function in apolipoprotein AI deficiency. J Clin Lipidol. 2019;13(3):468-480. e468.
Nelson AJ, Stephenson DJ, Bone RN, Cardona CL, Park MA, Tusing YG, Lei X, Kokotos G, Graves CL, Mathews CE. Lipid mediators and biomarkers associated with type 1 diabetes development. JCI Insight. 2020;5(16):e138034.
Szpigel A, Hainault I, Carlier A, Venteclef N, Batto AF, Hajduch E, Bernard C, Ktorza A, Gautier JF, Ferré P. Lipid environment induces ER stress, TXNIP expression and inflammation in immune cells of individuals with type 2 diabetes. Diabetologia. 2018;61(2):399–412.
Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus–atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5): 1835.
Brandts J, Tittel SR, Bramlage P, Danne T, Brix JM, Zimny S, Heyer CH, Holl RW, Müller-Wieland D. L ow-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in type 1 diabetes and type 2 diabetes: lipid goal attainment in a large German-Austrian diabetes registry. Diabetes Obes Metab. 2023;25(12):3700–8.
Toyoshima MTK, Santana MF, Silva AR, Mello GB, Santos-Bezerra DP, Goes MF, Bosco AA, Caramelli B, Ronsein GE, Correa-Giannella ML. Proteomics of high-density lipoprotein subfractions and subclinical atherosclerosis in type 1 diabetes mellitus: a case–control study. Diabetol Metab Syndr. 2023;15(1):42.
Della Pepa G, Lupoli R, Masulli M, Boccia R, De Angelis R, Gianfrancesco S, Piccolo R, Rainone C, Rivellese AA, Annuzzi G, Bozzetto L. Blood glucose control and metabolic dysfunction-associated steatotic liver disease in people with type 1 diabetes. J Endocrinol Invest. 2024:1–8. https://doi.org/10.1007/s40618-024-02333-2.
Acknowledgements
We are grateful to all the patients who took part in the present study and the whole endocrinology team, including doctors, nurses, and medical staff.
Funding
This work was supported by Training Project for Leading Talents of the Health and Family Planning Commission of Yunnan Province (grant numbers L-201624), Key Laboratory of Diabetes Immunology, Ministry of Education, National Clinical Research Center for Metabolic Diseases open project (grant numbers DKME202218), Yunnan Clinical Medical Research Center open project (grant numbers N2020LCZXKF-NM06), and Special Project for “Famous Doctor” of Yunnan Ten Thousand Talents Plan (grant numbers YNWR-MY-2019–020).
Author information
Authors and Affiliations
Contributions
Yunying Cai: Methodology, Writing-original draft preparation; Xiaojie Qi: Data curation, Writing-review & editing; Yongqin Zheng: Visualization, Project administration; Jie Zhang: Investigation, Supervision; Heng Su: Conceptualization, Funding acquisition.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was conducted in accordance with the regulations of the Medical Ethics Committee of the First People’s Hospital of Yunnan Province (No. KHLL2016-KY038). Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cai, Y., Qi, X., Zheng, Y. et al. Lipid profile alterations and biomarker identification in type 1 diabetes mellitus patients under glycemic control. BMC Endocr Disord 24, 149 (2024). https://doi.org/10.1186/s12902-024-01679-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01679-1