Abstract
Background
Chronic low-grade inflammation may mediate the relationship between obesity and diabetes, yet clinical research in this area remains scarce. Thus, this study aimed to explore the mediating role of chronic low-grade inflammation in this relationship using the National Health and Nutrition Examination Survey (NHANES).
Methods
This study involved 2,482 participants enrolled in the NHANES between 2005 and 2016. Based on the complex sampling survey weights of NHANES, logistic regression models were fitted, adjusting for various covariates to investigate the relationship between BMI, INFLA score, and diabetes. Moreover, weighted quantile sum (WQS) regression models were fitted to analyze the proportional contribution of individual components within the INFLA score. Finally, mediation analysis was conducted to quantitatively assess the magnitude of the mediating effect of the INFLA score on the relationship between BMI and diabetes.
Results
After adjusting for all potential confounding factors, a significant positive correlation was noted between INFLA score and diabetes [OR (95% CI), 1.038(1.003–1.075), p = 0.035]. Additionally, a significant positive correlation was observed between the high INFLA group and diabetes compared to the low INFLA group [OR (95% CI), 1.599(1.031–2.481), p = 0.037]. WQS regression models revealed that the proportional contributions of C-reactive protein, white blood cell count, platelet count, and neutrophil-to-lymphocyte ratio (NLR) were 55.5%, 34.8%, 8.46%, and 1.19%, respectively. Finally, the results of the mediation analysis indicated that the indirect effect of the INFLA score accounted for 10.20%.
Conclusions
Chronic low-grade inflammation was associated with diabetes and partially mediates the relationship between obesity and diabetes.
Similar content being viewed by others
Introduction
In recent years, the global prevalence of diabetes has been progressively increasing, emerging as a major public health concern [1, 2]. As of 2022, approximately 537 million people worldwide have been reported to be diagnosed with T2DM, with this number projected to increase by 46% by 2045 [3]. Consequently, diabetes and its related complications impose a significant economic burden on society and significantly affect the quality of life of patients [4, 5].
Obesity stands out as a primary risk factor for diabetes, with elevated BMI being associated with an increased risk of developing diabetes [6]. The potential mechanisms by which obesity leads to diabetes onset are diverse. Earlier research reported that obesity contributes to diabetes by disrupting the secretion of adipokines from adipose tissues [7]. Additionally, they documented that gut microbiota or virome may mediate the relationship between obesity and diabetes [8, 9].
As is well documented, obesity is often accompanied by increased oxidative stress, leading to elevated levels of inflammation [10]. An earlier study identified a close correlation between obesity and chronic low-grade inflammation [11]. The latter similarly affects the endocrine system, immune system, and metabolic processes, thereby predisposing individuals to various diseases such as diabetes and cardiovascular diseases [12, 13]. Basic research suggests that chronic low-grade inflammation mediates the effect of obesity on diabetes [14, 15]. However, clinical studies on this topic remain limited.
The National Health and Nutrition Examination Survey (NHANES) is a significant population health and nutritional status survey conducted by the government of the United States [16]. It employs random sampling nationwide and invites participants to undergo medical examinations, surveys, and nutritional assessments for data collection. The NHANES database contains data from multiple cycles, with each cycle typically lasting two years. The current study aimed to explore the mediating role of low-grade inflammation in the relationship between obesity and diabetes using the NHANES database.
Materials and methods
Study population
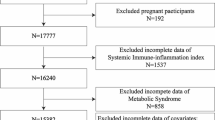
NHANES conducted OGTT testing on a subset of participants between 2005 and 2016. Out of a total of six cycles of data, a preliminary cohort of 60,936 participants was initially included in the study. Next, participants with missing data on fasting blood glucose levels, glycated hemoglobin levels, or OGTT were excluded (n = 50,853). Subsequently, individuals lacking any component data in the INFLA index were excluded (n = 6,729). Additionally, minors or participants with a history of malignant tumors were excluded (n = 723). Likewise, participants with extreme immunological values (< quartile Q1-3*interquartile (IQR) or > Q3 + 3*IQR) were excluded (n = 149). Ultimately, 2,482 participants were included in this study for further analysis (Fig. 1).
All participants had provided written informed consent. The NHANES project was approved by the Ethics Review Committee of the Centers for Disease Control and Prevention (CDC) in the United States.
Definition of BMI and obesity
BMI is defined as height ( cm ) divided by the square of weight ( kg ). In the regression analysis, using BMI as the exposure factor, patients were categorized into three groups stratified by BMI, namely the low BMI (≤ 25), medium BMI (25 ~ 30), and high BMI (> 30) groups. Obesity is measured by BMI and BMI over 30 is defined as obesity.
Definition of INFLA score
In the current study, the aggregated inflammation score (INFLA-score) was regarded as a comprehensive indicator of low-grade inflammation in the body. The INFLA score is composed of four components (CRP, WBC count, platelet count, and neutrophil-to-lymphocyte ratio (NLR)), which are considered synergistic inflammatory markers. The specific method for calculating the INFLA score involves dividing the four components into deciles, with values ranging from + 1 to + 4 assigned to deciles 7 to 10 and values ranging from − 4 to -1 assigned to deciles 1 to 4. All values are then summed, resulting in an INFLA score ranging from − 16 to + 16, where higher scores reflect higher levels of low-grade inflammation [17].
The measurement methods and procedures for all biochemical indicators are detailed in the supplementary materials.
Definition of diabetes
According to the diagnostic criteria of the American Diabetes Association (ADA), diabetes is defined by self-reported diagnosis, fasting blood glucose level (FBG) ≥ 126 mg/dL, hemoglobin A1c level (HbA1c) level ≥ 6.5%, or a two-hour blood glucose level during an oral glucose tolerance test (OGTT) ≥ 200 mg/dL [18]. Ultimately, based on these criteria, diabetes can be categorized as a binary variable.
Definition of covariates
Information on various demographic and health-related factors was collected from the NHANES Household Interview, including age, gender, race/ethnicity, educational level, household income, alcohol consumption status, and physical examination. Race was categorized as White and Other, whilst educational level was classified as college/university degree or below. Household income and poverty ratio were divided into 0–3.0 or > 3.0. Drinkers were defined as individuals who consumed at least 12 alcoholic drinks within a year. Systolic and diastolic blood pressures were calculated by the average of three measurements. Hypertension was defined as a systolic blood pressure of ≥ 140mmHg, a diastolic blood pressure of ≥ 90mmHg, or a self-reported diagnosis of hypertension.
Clinical indicators such as serum cotinine, triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl translerase (GGT), uric acid (UA), and creatinine (CR) were quantified in the NHANES laboratory. Serum cotinine is the primary metabolite formed after nicotine metabolism in the body, accurately reflecting an individual’s tobacco exposure level, including both active and passive smoking, and thus providing a reliable and objective measure of smoking levels [19].
Statistical analysis
All statistical analyses were conducted using Python 3.10.13 and R 4.2.1. A two-tailed P < 0.05 was considered statistically significant. Based on the description of baseline variables, the entire population was grouped and pooled, including the non-diabetic population and the diabetic population. Continuous variables following a normal distribution were presented as mean ± standard deviation, while categorical variables were expressed as numbers (proportions). One-way analysis of variance was employed to compare continuous variables in order to assess baseline characteristic differences between the diabetic and non-diabetic populations, whereas Pearson’s chi-square test was used to compare categorical variables.
To examine the association between BMI, INFLA score, and diabetes, univariate regression analysis was first performed to preliminarily examine the relationships among the three variables. Furthermore, three multivariable regression models adjusted for different covariates were generated. Model 1 was exclusively adjusted for demographic factors (age, gender, race, educational level, and poverty income ratio). Meanwhile, model 2 included adjustments from Model 1 plus alcohol consumption, serum cotinine levels, systolic blood pressure, diastolic blood pressure, and BMI. Finally, model 3 was further adjusted for laboratory biochemical indicators, including TG, LDL, HDL, ALT, AST, GGT, UA, and CR. To mitigate the impact of multicollinearity, the variance inflation factor (VIF) was determined, with variables having a VIF over 5 considered to exhibit multicollinearity and thus excluded. Considering the complex sampling design of NHANES, sample weights, clustering, and stratification were incorporated, which are necessary for analyzing NHANES data.
Stratified analyses were also carried out based on age, gender, race, educational level, and poverty-income ratio. Moreover, interaction tests were performed to identify significant interactions between the different subgroups.
Weighted quantile sum (WQS) regression analysis was employed to assess the contribution of each component in the composite index to its effect. Its advantage lies in its ability to account for potential interactions between components in the mixture and provide insights into components contributing more significantly to health effects. 50% of the data was assigned to the training set, and the remaining 50% of the data was assigned to the validation set. Then, both positive and negative WQS regression analyses were conducted, and component weights and the overall significance P-values were determined using bootstrap resampling.
Finally, a mediation effect analysis was conducted, with BMI as the exposure factor, diabetes as the outcome factor, and INFLA as the mediator factor. The proportions of total, direct, and indirect effects were calculated. Bootstrap resampling was used to examine the significance of the mediation effects.
Results
Participant characteristics
Among all 2,482 participants with an average age of 48.24 years, 51.93% of whom were females, 241 were diabetics, yielding a prevalence rate of approximately 9.71%… The majority of participants were Caucasian (78.44%), and the distribution of educational levels between those with a college degree or higher and those below was roughly comparable.
The age of participants in the diabetes group was higher, and these patients were more likely to be male. Additionally, diabetes patients had lower educational levels, higher BMI, and higher systolic blood pressure. Similarly, the levels of TG, ALT, GGT, UC, and CR were higher in the diabetes group, whereas the level of HDL was lower. Lastly, the INFLA score was significantly higher in the diabetes group compared to the non-diabetes group. All these differences were statistically significant. In contrast, race and poverty ratio were similar between the two groups (Table 1).
Association between BMI, INFLA, and diabetes
In the regression analysis, using BMI as the exposure factor, patients were categorized into three groups stratified by BMI, namely the low BMI (≤ 25), medium BMI (25 ~ 30), and high BMI (> 30) groups. Continuous variables and categorical variables of BMI were individually regressed against the outcome of INFLA using single-factor linear regression and against the binary outcome of diabetes using logistic regression. Interestingly, the results indicated a significant positive correlation between BMI and both INFLA [Beta (95% CI), 0.327(0.279–0.374), p < 0.001] and diabetes [OR (95% CI), 1.090(1.071–1.109),p < 0.001]. As anticipated, significant positive correlations were also observed in the medium and high BMI groups relative to the low BMI group.
Furthermore, in the regression analysis using INFLA as the exposure factor, patients were stratified based on INFLA tertiles: low INFLA (≤-3), medium INFLA (-3 ~ 3), and high INFLA (> 3). When diabetes was considered the binary outcome, a significant positive correlation was detected with INFLA [OR (95% CI), 1.072(1.041–1.104), p < 0.001]. Additionally, a significant positive correlation was observed in the high INFLA group compared to the low INFLA group [OR (95% CI), 2.485(1.662–3.716), p < 0.001]. (See Table 2).
To mitigate the potential confounding effects, a multivariable logistic regression model was constructed. Initially, variance inflation factors (VIFs) were computed, and variables with VIFs exceeding 5 were excluded. Consequently, in Model 2, the variable diastolic blood pressure was excluded, and the variable AST was excluded in Model 3. Ultimately, after adjusting for all covariates, a significant positive correlation was detected between INFLA score and diabetes [OR (95% CI), 1.038(1.003–1.075), p = 0.035]. Lastly, a significant positive correlation was also noted in the high INFLA group relative to the low INFLA group [OR (95% CI), 1.599(1.031–2.481), p = 0.037]. (See Table 3).
Results of subgroup analysis
To elucidate the disparities in the aforementioned findings across the population groups, subgroup analyses were conducted based on various demographic factors, comprising age, gender, race, educational level, and poverty-income ratio. In other words, regression analyses were re-performed by introducing interaction terms and adjusting for specific covariates. Age was stratified into three groups: 20–40 years, 41–60 years, and above 60 years. After adjusting for all covariates except for the stratification variable, the results revealed a consistent positive correlation between INFLA score and the outcome of diabetes across the age groups, genders, educational levels, and poverty income ratios in the overall population, with no significant interaction effects observed (P-interaction > 0.05). Furthermore, there was an interaction effect between race and INFLA score (P-interaction < 0.05). Specifically, a stronger positive correlation was found in the subgroup of individuals of White race compared to the overall population [OR (95% CI), 1.047(1.008–1.087), p = 0.019], whereas this association was not significant in other racial subgroups (p-value > 0.05). (See Table 4 for details).
Results of WQS model
Furthermore, the WQS model was established to analyze the weights of the four components in the INFLA score. After adjusting for all covariates, the results showed that C-reactive protein (55.5%) and white blood cell count (34.8%) accounted for the majority of the weight, whereas the contribution of platelet count (8.46%) and neutrophil-to-lymphocyte ratio (NLR) (1.19%) was comparatively lower. The positive WQS model was significant (P = 0.014), whereas the negative WQS model was not significant (P > 0.05). (See Fig. 2; Table 5).
Results of mediation analysis
The results of the mediation analysis signaled that the INFLA score partially mediated the relationship between BMI and diabetes. Specifically, after adjusting for all covariates, the total effect (βtotal effect) was 9.49 × 10− 4, the direct effect (βADE) was 8.52 × 10− 4, the indirect effect (βACME) was 9.67 × 10− 5, and the proportion of the indirect effect was 10.20%. Noteworthily, both the direct and indirect effects were statistically significant (P < 0.05). (Refer to Fig. 3; Table 6).
Sensitivity analysis
The binary classification of participants’ hypertension status was incorporated as a covariate in the regression model to avoid bias in representing participants’ blood pressure status solely by including systolic blood pressure. The results demonstrated that even after substituting systolic blood pressure with hypertension status, the statistical association between IFNLA and diabetes remained significant [OR (95% CI), 1.039(1.003–1.077), p = 0.034] (see sTable S1).
Discussion
This study utilized the NHANES database to explore the association between BMI, INFLA score, and diabetes. The results revealed that for each unit increase in the INFLA score, there was a corresponding 3.8% increase in the prevalence of diabetes. Additionally, the prevalence of diabetes in the high INFLA group was 59.9% higher than in the low INFLA group. Furthermore, the mediation analysis determined that INFLA partially mediated the relationship between BMI and diabetes, with the indirect effect accounting for 10.2% of the association.
The association between chronic low-grade inflammation and diabetes noted in this study is consistent with the findings of previous studies. Past studies have indicated that elevated CRP levels are associated with an increased risk of type 2 diabetes, and thus, CRP can serve as a predictive factor for the development of diabetes [20, 21]. Ascribed to its relative stability and ease of measurement, CRP is commonly used as a marker for assessing systemic inflammation in humans [22]. However, a single marker may not fully reflect an individual’s level of chronic low-grade inflammation [23]. In the present study, the WQS regression model demonstrated that CRP accounted for about half of the weight of the INFLA score, whilst the other half consisted of the other three components. Elevated Plt, WBC, and NLR are generally used as indicators of cellular inflammation in epidemiological studies [24, 25].
INFLA scores are frequently associated as intermediate factors with psychological disorders such as anxiety and depression [26, 27] and have been shown to mediate the association between acrylamide biomarkers and cancer mortality [28]. Additionally, it is well-established that obesity increases the level of chronic low-grade inflammation and can impair physiological function by mediating chronic low-grade inflammation [29, 30]. However, clinical studies on obesity mediating the occurrence of diabetes through chronic low-grade inflammation are currently lacking.
Multiple fundamental studies have evinced that obesity can drive chronic systemic inflammation, ultimately resulting in type 2 diabetes (T2D) [31]. The mechanisms by which inflammation mediates obesity-induced diabetes may be diverse. The enlarged adipose tissue leads to hypoxia due to inadequate perfusion and increased oxygen demand, which in turn induces inflammation. Substantial evidence suggests a direct correlation between inflammation and insulin resistance [32]. Additionally, obesity can promote the accumulation of immune cells, cytokines, and chemokines, eliciting pancreatic inflammation and impairing insulin secretion [31]. Obesity also leads to a significant increase in recruited hepatic macrophages, as well as local synthesis of inflammatory chemokines and cytokines, resulting in hepatic insulin resistance [14]. Prior research also suggested that obesity-induced dysbiosis of the gut microbiota triggers low-grade chronic inflammation, thereby exerting a cascade effect on insulin resistance [33]. In summary, the pathways through which obesity leads to diabetes are diverse, with chronic low-grade inflammation playing a prominent role.
To the best of our knowledge, this is the first study exploring the mediating effect of low-grade inflammation on the relationship between BMI and diabetes based on a large-scale database. Using the INFLA score enabled a more objective assessment of individuals’ systemic chronic inflammation levels. We employed a curated dataset compiled from previous studies, which combined all files of NHANES III (1988–1994) and Continuous NHANES (1999–2018), taking into account variations in variable nomenclature and measurement units, thereby partially mitigating the effects of heterogeneity across different years. Furthermore, the complex weights of the stratified sampling of NHANES were incorporated and applied, thus providing a more accurate reflection of the overall population.
However, this study has several limitations that merit acknowledgment. Firstly, our research relied on cross-sectional data, which precludes establishing causal relationships between the investigated factors. Secondly, the NHANES database included only the U.S. population, thus the study findings may not be generalizable to other populations. Indeed, these results necessitate further validation among other racial groups. Lastly, despite our efforts to adjust for various confounding factors during the data analysis process, the inherent limitations of observational studies prevented the exclusion of other potential influences.
Conclusions
In summary, our study identified a relationship between INFLA score and diabetes, with the INFLA score partially mediating the relationship between BMI and diabetes. Despite numerous studies focusing on related mechanisms, further cohort studies are necessitated to validate our results.
Data availability
The data used in this study can be publicly accessed at https://www.kaggle.com/datasets/nguyenvy/nhanes-19882018?select=dictionary_nhanes.csv.
References
Ruze R et al. Apr. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Frontiers in endocrinology vol. 14 1161521. 21 2023, https://doi.org/10.3389/fendo.2023.1161521.
Tinajero MG, Vasanti S, Malik. An update on the epidemiology of type 2 diabetes: A Global Perspective. Endocrinol Metabolism Clin North Am vol. 2021;50(3):337–55. https://doi.org/10.1016/j.ecl.2021.05.013.
Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Reviews Cardiol vol. 2023;20(10):685–95. https://doi.org/10.1038/s41569-023-00877-z.
Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21(1):74. Published 2022 May 14. https://doi.org/10.1186/s12933-022-01516-6.
Teck J. Diabetes-Associated Comorbidities. Primary care vol. 49,2 (2022): 275–286. https://doi.org/10.1016/j.pop.2021.11.004.
Maggio CA, Xavier Pi-Sunyer F. Obesity and type 2 diabetes. Endocrinology and metabolism clinics of North America vol. 32,4 (2003): 805 – 22, viii. https://doi.org/10.1016/s0889-8529(03)00071-9.
Saxton SN, et al. Mechanistic links between obesity, diabetes, and blood pressure: role of Perivascular Adipose tissue. Physiological Reviews vol. 2019;99(4):1701–63. https://doi.org/10.1152/physrev.00034.2018.
Pitocco D, et al. The role of gut microbiota in mediating obesity and diabetes mellitus. Eur Rev Med Pharmacol Sci Vol. 2020;24(3):1548–62. https://doi.org/10.26355/eurrev_202002_20213.
Yang K et al. Alterations in the gut virome in obesity and type 2 diabetes Mellitus. Gastroenterology 161,4 (2021): 1257–e126913. https://doi.org/10.1053/j.gastro.2021.06.056.
Codoñer-Franch P, et al. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Translational Research: J Lab Clin Med vol. 2011;158(6):369–84. https://doi.org/10.1016/j.trsl.2011.08.004.
Kawai T, et al. Adipose tissue inflammation and metabolic dysfunction in obesity. American journal of physiology. Cell Physiol vol. 2021;320(3):C375–91. https://doi.org/10.1152/ajpcell.00379.2020.
Scheithauer TPM et al. Oct. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Frontiers in immunology vol. 11 571731. 16 2020, https://doi.org/10.3389/fimmu.2020.571731.
Silveira Rossi L et al. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/metabolism research and reviews vol. 38,3 (2022): e3502. https://doi.org/10.1002/dmrr.3502.
Saltiel AR, Jerrold M, Olefsky. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest Vol. 2017;127(1):1–4. https://doi.org/10.1172/JCI92035.
Rohm TV et al. Inflammation in obesity, diabetes, and related disorders. Immunity 55,1 (2022): 31–55. https://doi.org/10.1016/j.immuni.2021.12.013.
NCHS. National Health and Nutrition Examination Survey. Published 2022. https://www.cdc.gov/nchs/nhanes/index.htm.
Bonaccio M et al. A score of low-grade inflammation and risk of mortality: prospective findings from the Moli-Sani study. Haematologica 101,11 (2016): 1434–41. https://doi.org/10.3324/haematol.2016.144055.
Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279. https://doi.org/10.1186/s12933-023-02030-z. Published 2023 Oct 17.
Zhang Y, et al. Self-reported smoking, serum cotinine, and blood DNA methylation. Environ Res. 2016;146:395–403. https://doi.org/10.1016/j.envres.2016.01.026.
Pradhan AD et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286,3 (2001): 327–34. https://doi.org/10.1001/jama.286.3.327.
Thorand B, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Archives Intern Med vol. 2003;163(1):93–9. https://doi.org/10.1001/archinte.163.1.93.
Danesh J, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New Engl J Med vol. 2004;350(14):1387–97. https://doi.org/10.1056/NEJMoa032804.
Gialluisi A, et al. Lifestyle and biological factors influence the relationship between mental health and low-grade inflammation. Brain Behav Immun. 2020;85:4–13. https://doi.org/10.1016/j.bbi.2019.04.041.
Bonaccio M et al. Adherence to the Mediterranean diet is associated with lower platelet and leukocyte counts: results from the Moli-Sani study. Blood 123,19 (2014): 3037–44. https://doi.org/10.1182/blood-2013-12-541672.
Huang Z, et al. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med vol. 2020;38(3):641–7. https://doi.org/10.1016/j.ajem.2019.10.023.
Shi H et al. Low-grade inflammation as mediator between diet and behavioral disinhibition: a UK Biobank study. Brain, behavior, and immunity 106 (2022): 100–10. https://doi.org/10.1016/j.bbi.2022.07.165.
Cen M et al. Associations between metabolic syndrome and anxiety, and the mediating role of inflammation: findings from the UK Biobank. Brain, behavior, and immunity 116 (2024): 1–9. https://doi.org/10.1016/j.bbi.2023.11.019.
Gu W, et al. The association between biomarkers of acrylamide and cancer mortality in U.S. adult population: evidence from NHANES 2003–2014. Frontiers in oncology. 28 Sep. 2022;12 970021. https://doi.org/10.3389/fonc.2022.970021.
Norris T, et al. Obesity in early adulthood and physical functioning in mid-life: investigating the mediating role of c-reactive protein. Brain Behav Immun. 2022;102:325–32. https://doi.org/10.1016/j.bbi.2022.03.008.
Kemalasari I, et al. Effect of calorie restriction diet on levels of C reactive protein (CRP) in obesity: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metabolic Syndrome vol. 2022;16(3):102388. https://doi.org/10.1016/j.dsx.2022.102388.
Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55(1):31–55. https://doi.org/10.1016/j.immuni.2021.12.013.
Lee YS, Kim JW, Osborne O, et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–52. https://doi.org/10.1016/j.cell.2014.05.012.
Patterson E, et al. Gut microbiota, obesity and diabetes. Postgrad Med J Vol. 2016;92(1087):286–300. https://doi.org/10.1136/postgradmedj-2015-133285.
Acknowledgements
We want to acknowledge the available NHANES data.
Funding
This research has no funder.
Author information
Authors and Affiliations
Contributions
L.K. and L.J.B. designed this experiment.L.P. wrote the manuscript.Q.Q.C. conducted statistical analysis.N.C.Y. carried out manuscript revision and language polishing.W.C. and G.X.H. conducted literature search.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The National Health and Nutrition Examination Survey (NHANES) received approval from the Institutional Review Board of the National Center for Health Statistics. Written informed consent was obtained from all participants during the national survey conducted in the United States. All files are available in the supplementary materials.
Consent for publication
Not applicable.
Disclosure
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, P., Qiao, Q., Nie, C. et al. The mediating role of chronic low-grade inflammation participation in the relationship between obesity and type 2 diabetes: findings from the NHANES. BMC Endocr Disord 24, 130 (2024). https://doi.org/10.1186/s12902-024-01671-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01671-9