Abstract
Background
Arginine vasopressin deficiency (AVP-D) can occur due to various conditions, so clarifying its cause is important for deciding treatment strategy. Although several cases of AVP-D following coronavirus disease 2019(COVID-19) infection or COVID-19 vaccination have been reported, the diagnosis of the underlying disease has not been reported in most cases.
Case presentation
A 75-year-old woman who presented with polydipsia and polyuria 9 weeks after contracting COVID-19 and 5 weeks after receiving the SARS-CoV-2 vaccination, leading to the final diagnosis of AVP-D 8 months after the first appearance of symptoms. Interestingly, pituitary magnetic resonance imaging (MRI) still revealed stalk enlargement frequently observed in patients with SARS-CoV-2 vaccination-induced AVP-D. Although this finding could not rule out any malignancies, we additionally measured anti-rabphilin-3A antibodies, a known marker for lymphocytic infundibulo-neurohypophysitis (LINH), and found that the results were positive, strongly suggesting LINH as the cause of this disease. Thus, we avoided pituitary biopsy. At the follow-up MRI conducted 12 months after the initial consultation, enlargement of the pituitary stalk was still observed.
Conclusion
We experienced a case with LINH probably induced by SARS-CoV-2 vaccination. In SARS-CoV-2 vaccination-related LINH, unlike typical LINH, there is a possibility of persistent pituitary stalk enlargement on MRI images for an extended period, posing challenges in differential diagnosis from other conditions. Pituitary stalk enlargement and positive anti-rabphilin-3A antibodies may help in the diagnosis of AVP-D induced by SARS-CoV-2 vaccination.
Similar content being viewed by others
Background
Arginine vasopressin deficiency (AVP-D), formerly known as central diabetes insipidus, is known to occur due to various causes such as malignant tumors, surgery, and inflammatory conditions. The correct diagnosis of the underlying disease in each case is important for deciding the treatment strategy [1]. Lymphocytic infundibulo-neurohypophysitis (LINH) is one of autoimmune disease and induces AVP-D. In recent years, anti-rabphilin-3A antibodies, which are autoantibodies targeting rabphilin-3A, a specific GTP-Rab3A binding protein present in the secretory granules of the posterior pituitary, have been identified as highly sensitive markers for LINH [2].
The onset of various endocrine disorders such as type 1 diabetes mellitus, subacute thyroiditis, Graves’ disease, and anterior pituitary inflammation that are considered to be related to autoimmunity have been reported following coronavirus disease 2019 (COVID-19) infection [3]. Additionally, the onset of such diseases has been documented following SARS-CoV-2 vaccination [4]. While some cases of developing pituitary inflammation and the consequent AVP-D following COVID-19 infection [5,6,7,8,9,10,11] or SARS-CoV-2 vaccination [12,13,14,15,16,17,18] were reported, the cases with proved detailed involvement of autoimmunity as the cause of these diseases were rare.
Here we report a case of a 75-year-old woman who developed AVP-D with positive anti-rabphilin-3A antibodies. The onset occurred 9 weeks after contracting COVID-19 infection and 5 weeks after receiving SARS-CoV-2 vaccination.
Case presentation
A 75-year-old woman with no apparent medical history or family history contracted COVID-19 infection on December 10, 2022, which resolved without the need for hospitalization, and initially, no sequelae were noted. Additionally, she had received four SARS-CoV-2 vaccinations: the first dose on July 10, 2021 (tozinameran; Pfizer-BioNTech, Lot No. FC9880), the second dose on July 31, 2021 (tozinameran; Pfizer-BioNTech, Lot No. EY0583), the third dose on March 10, 2022 (elasomeran; Takeda/Moderna, Lot No. 000048 A), and the fourth dose on August 17, 2022 (tozinameran; Pfizer-BioNTech, Lot No. FR1790). On January 5, 2023, she received her fifth dose of SARS-CoV-2 vaccination (tozinameran/famtozinameran; Pfizer-BioNTech, Lot No. GJ7139). Around February 10, she began experiencing polydipsia and polyuria. Although her primary care physician found no abnormalities on physical examination, she was referred to a post-COVID-19 sequelae outpatient clinic due to suspected psychogenic diseases, where she underwent further examination as an inpatient. During hospitalization, her water intake was approximately 3–4 L per day, and urine output was around 3 L per day, raising suspicion of AVP-D, which led to her admission to our hospital on October 2, 2023. Upon admission, she had a height of 146 cm and a weight of 56.3 kg, with no signs of dryness in the oral cavity. Her 24-hour urine output was 3900 mL/day, resulting in a urine volume per body weight of 69.2 mL/kg, indicating polyuria. Blood tests at admission revealed a serum sodium level of 146 mEq/L, an AVP concentration of 0.6 ng/mL indicating its decreased secretion, and a urine osmolarity of 224 mOsm/L (Table 1). Baseline hypothalamic-pituitary-adrenal axis values upon admission showed ACTH 15.7 pg/mL (reference range 7.2–63.3 pg/mL), cortisol 5.9 µg/dL (reference range 5.1–23.6 µg/dL). Both the three-part loading test (Table 2) and GH releasing peptide-2 (GHRP-2) loading test (Table 3) showed that the anterior pituitary hormone reserve was maintained.
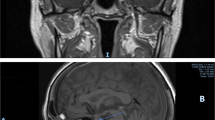
In the hypertonic saline infusion test, the responsiveness of plasma AVP decreased compared to the increase in serum Na levels. Additionally, after 120 min of the hypertonic saline infusion test, 5 units of exogenous vasopressin were administered, resulting in an increase in urinary osmolarity (from 322 to 491 mOsm/kg) and a decrease in urine output (from 5.4 to 3.5 mL/kg/hr) compared to pre-administration levels (Table 4). Subsequent administration of desmopressin (DDAVP) resulted in an increase in urine osmolarity, leading to the diagnosis of AVP-D. Serum levels of human chorionic gonadotropin β-subunit (β-hCG), α-fetoprotein (AFP), angiotensin I-converting enzyme (ACE), and immunoglobulin G4 (IgG4) were within normal ranges, and proteinase-3-antineutrophil cytoplasmic antibody (PR3-ANCA) was negative (Table 1). MRI conducted for further investigation revealed loss of posterior lobe high signal intensity on T1-weighted images (Fig. 1A, B). Contrast-enhanced MRI demonstrated a thickened pituitary stalk measuring 3.5 mm with strong enhancement (Fig. 1C, D).
Pituitary magnetic resonance imaging (MRI) at first visit. (A) Sagittal view of plain MRI. (B) Coronal view of plain MRI. No high signal was observed in the posterior pituitary lobe. (C) Sagittal view of contrast-enhanced MRI. (D) Coronal view of contrast-enhanced MRI. Enlargement of the pituitary stalk (yellow arrowhead) and enhancement are evident. No pituitary tumor was observed
Based on these findings, lymphocytic infundibulo-neurohypophysitis (LINH) was initially suspected. However, since the thickening of the pituitary stalk persisted even 8 months after symptom onset and it was atypical, we considered to perform a pituitary biopsy for histological confirmation. However, due to the patient’s advanced age and her desire to minimize surgical invasiveness, we opted to measure anti-rabphilin-3A antibodies, which are considered specific to LINH and the presence of serum anti-rabphilin-3A antibody was detected by western blotting (Fig. 2). It supported the diagnosis of LINH.
Measurement of anti-rabphilin-3A antibodies by Western blotting. Detection of anti-rabphilin-3A antibodies by Western blotting. Recombinant full-length human rabphilin-3A expressed in HEK293FT cells (RPH3A) or negative control (-) were probed with serum from the present case (patient) or from a patient who was diagnosed with LINH by biopsy previously (positive control patient). Recombinant full-length human rabphilin-3A expressed in HEK293FT cells was also probed with an anti-V5 antibody as a positive control (Anti-V5 antibody) in the first lane from the left. Arrowheads indicate the presence of anti-rabphilin-3A antibodies. Dashed arrowheads indicate the absence of anti-rabphilin-3A antibodies. A protein band of 76 kDa that appeared in RPH3A but not in that of negative control was considered to be positive for anti-rabphilin-3A antibodies. RPH3A, rabphilin-3A, TFs, transfections of full-length human rabphilin-3A gene
For the treatment of AVP-D, oral DDAVP 60 µg/day was initiated, resulting in a decrease in urine output, and the patient continues to attend outpatient visits. We considered pharmacological doses of steroid treatment for hypophysitis, but we did not implement steroid therapy due to the absence of clinical symptoms such as headache or visual field disturbances, the limited expected efficacy in treating AVP-D, and concerns regarding the side effects of steroid administration in elderly patients. MRI imaging 12 months after the initial consultation did not detect new lesions persisted with stalk enlargement suggesting a stable course of LINH not likely any malignancies (Fig. 3A, B).
Materials and methods
Western blotting was conducted on serum obtained from the patient for detection of anti-rabphilin-3A antibodies, as reported previously [2]. A vector containing the full-length human rabphilin-3A gene was transfected into HEK293FT cells to produce recombinant human rabphilin-3A protein. Expression of recombinant human rabphilin-3A protein was confirmed using an anti-V5 antibody. As a negative control, the same vector without the rabphilin-3A gene was transfected into HEK293FT cells. A protein band presenting a size of 76 kDa, which corresponds to the molecular weight of rabphilin-3A, appeared in the lysate of cells transfected with the rabphilin-3A protein but not in that of control cells, which was considered to be positive for anti-rabphilin-3A antibodies, as reported previously.
Discussion and conclusions
The insights gained from this case indicate that measuring anti-rabphilin-3A antibodies is helpful to diagnosed LINH in cases with AVP-D occurring after COVID-19 infection and/or SARS-CoV-2 vaccination. Furthermore, in our case, pituitary stalk thickening can persist for over 8 months.
Hypophysitis has various etiologies, including lymphocytic, granulomatous, xanthomatous, necrotizing, IgG4-related systemic disease, and chemotherapy agents, apart from pregnancy-related causes. Therefore, the gold standard for the final diagnosis of hypophysitis including LINH is pituitary biopsy, however high patient invasiveness is a major clinical concern [19].
Sheen et al. recommend biannual follow-up pituitary MRI scans for a duration of two years in cases demonstrating pituitary stalk thickening to monitor the progression of the disease [20].
In our case, pituitary stalk thickening persisted even at 8 months after symptom onset, prompting the consideration of a pituitary biopsy for a definitive diagnosis of LINH, also to rule out malignant conditions such as Langerhans cell histiocytosis. However, the evaluation of anti-rabphilin-3A antibodies targeting antigens specific to the posterior pituitary which are highly sensitive markers for LINH [21] was helpful for avoiding invasive pituitary biopsies.
Previous case reports suggest that both COVID-19 infection and SARS-CoV-2 vaccination could potentially trigger the onset of AVP-D.
There are seven reports of pituitary dysfunction accompanied by AVP-D following SARS-CoV-2 vaccination [12,13,14,15,16,17,18]. The majority of these cases were female, four cases presented only with AVP-D, and the time from vaccination to disease onset varied from 3 days to 8 weeks post-vaccination. [14,15,16,17,18]. Interestingly, pituitary stalk enlargement was frequently detected on MRI scans in 85% (6/7) (Table 5).
On the other hand, there are also seven reports of pituitary dysfunction accompanied by AVP-D after COVID-19 infection. Among the seven cases, only one case exhibited symptoms of pituitary anterior lobe dysfunction other than AVP-D. the time from infection to disease onset varied from 2 to 8 weeks. Unlike SARS-CoV-2 vaccination-related AVP-D, thickening of the pituitary stalk was only observed in 17% (1/6), (Table 6) [5,6,7,8,9,10,11]. In reports of long-term follow-up of idiopathic cases of AVP-D, 79.1% (34 of 43 patients) were detected to have pituitary stalk enlargement on MRI at the time of onset, and 93.0% (40 of 43 patients) up to 6 months thereafter [22]. Considering the frequency of pituitary stalk enlargement in both idiopathic AVP-D and SARS-CoV-2 vaccination-related AVP-D, it is presumed that SARS-CoV-2 vaccination-related AVP-D shares a similar pathophysiology with idiopathic AVP-D.
One potential contributor to pituitary inflammation following vaccination is the adjuvant-induced autoimmune (ASIA) syndrome, which is believed to overreact immune responses using adjuvants [23].
However, AVP-D due to vaccines other than the SARS-CoV-2 has been limited only with the older smallpox vaccine [24, 25], with no recent reports from other vaccines, suggesting a possible specific effect of adjuvants in the SARS-CoV-2 vaccine. Secondly, antigen cross-reactivity between SARS-CoV-2 spike protein antibodies and tissue proteins such as thyroid peroxidase protein has been known [26]. Although it is unknown for pituitary and hypothalamic cells, these cells may possess cross-reactivity to induce autoimmunity.
On the other hand, the following mechanism is considered for the development of AVP-D during COVID-19 infection: SARS-CoV-2 utilizes its spike protein to invade host cells via the Angiotensin converting enzyme 2 (ACE2) receptor [27]. The hypothalamus expresses ACE2 receptors and is thought to be a target for SARS-CoV-2, which may lead to AVP-D [28].
COVID-19 infection-related AVP-D may differ from idiopathic AVP-D in terms of its pathophysiology and imaging characteristics, such as those seen in LINH. There has been only one case of anti-rabphilin-3A antibody-positive AVP-D without pituitary stalk enlargement, which was considered to have resolved due to its chronic nature [29]. It is possible that this case did not initially present with pituitary enlargement. The measurement of anti-rabphilin-3A antibody has been predominantly conducted in cases with imaging characteristics. However, accumulation of cases for antibody measurements are needed for further investigation of the underlying causes, including COVID-19 infection-related AVP-D and cases without other imaging characteristics, to facilitate the advancement of etiological assessment.
In conclusion, it was presumed that the SARS-CoV-2 vaccine was the triggering factor in our case. Pituitary stalk enlargement and positive anti-rabphilin-3A antibodies may help in the diagnosis of AVP-D induced by SARS-CoV-2 vaccination. As testing for anti-rabphilin-3A antibodies becomes more widespread and data accumulates, it is anticipated that it will contribute to clarify the detail causes of such conditions.
We reported this adverse event related to the vaccine to Pfizer Japan.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- ACTH:
-
Adrenocorticotropic hormone
- AVP:
-
Arginine vasopressin
- COVID-19:
-
Coronavirus disease 2019
- DDAVP:
-
Desmopressin
- GHRP-2:
-
Growth hormone-releasing peptide-2
- LINH:
-
Lymphocytic infundibulo-neurohypophysitis
- MRI:
-
Magnetic resonance imaging
References
Tomkins M, Lawless S, Martin-Grace J, Sherlock M, Thompson CJ. Diagnosis and management of Central Diabetes Insipidus in adults. J Clin Endocrinol Metab. 2022;107(10):2701–15.
Arihara Z, Sakurai K, Niitsuma S, Sato R, Yamada S, Inoshita N, Iwata N, Fujisawa H, Watanabe T, Suzuki A, et al. Studies on anti-rabphilin-3A antibodies in 15 consecutive patients presenting with central diabetes insipidus at a single referral center. Sci Rep. 2022;12(1):4440.
Mirza SA, Sheikh AAE, Barbera M, Ijaz Z, Javaid MA, Shekhar R, Pal S, Sheikh AB. COVID-19 and the Endocrine System: a review of the current information and misinformation. Infect Dis Rep. 2022;14(2):184–97.
Zhao Y. X Wu 2022 Influence of COVID-19 vaccines on endocrine system. Endocrine 78 2 241–6.
Rajevac H, Bachan M, Khan Z. Diabetes insipidus as a Symptom of Covid-19 infection: Case Report. Chest. 2020;158(4):a2576–2576.
Sheikh AB, Javed N, Sheikh AAE, Upadhyay S, Shekhar R. Diabetes insipidus and concomitant myocarditis: a late sequelae of COVID-19 infection. J Investig Med High Impact Case Rep. 2021;9:2324709621999954.
Misgar RA, Rasool A, Wani AI, Bashir MI. Central diabetes insipidus (Infundibuloneuro hypophysitis): a late complication of COVID-19 infection. J Endocrinol Invest. 2021;44(12):2855–6.
Sheikh AB, Javaid MA, Sheikh AAE, Shekhar R. Central adrenal insufficiency and diabetes insipidus as potential endocrine manifestations of COVID-19 infection: a case report. Pan Afr Med J. 2021;38:222.
Yavari A, Sharifan Z, Larijani B, Mosadegh Khah A. Central diabetes insipidus secondary to COVID-19 infection: a case report. BMC Endocr Disord. 2022;22(1):134.
Lizzi M, Arico M, Carlone G, Anzellotti MT, Trotta D, Palatino V. Central Diabetes Insipidus: another rare complication of SARS-CoV-2 infection in children? Pediatr Infect Dis J. 2022;41(10):e448.
Suresh Kumar S, Kumar K, Venkataramani S, Ghazi NM. Central Diabetes Insipidus: an Acute Manifestation of COVID-19 infection. Cureus. 2023;15(8):e43884.
Murvelashvili N, Tessnow A. A case of Hypophysitis following immunization with the mRNA-1273 SARS-CoV-2 vaccine. J Investig Med High Impact Case Rep. 2021;9:23247096211043386.
Ankireddypalli AR, Chow LS, Radulescu A, Kawakami Y, Araki T. A case of Hypophysitis Associated with SARS-CoV-2 vaccination. AACE Clin Case Rep. 2022;8(5):204–9.
Bouca B, Roldao M, Bogalho P, Cerqueira L, Silva-Nunes J. Central Diabetes Insipidus following immunization with BNT162b2 mRNA COVID-19 vaccine: a Case Report. Front Endocrinol (Lausanne). 2022;13:889074.
Ach T, Kammoun F, Fekih HE, Slama NBH, Kahloun S, Fredj FB, Laouani C, Ach K. Central diabetes insipidus revealing a hypophysitis induced by SARS-CoV-2 vaccine. Therapie. 2023;78(4):453–5.
Ishay A, Shacham EC. Central diabetes insipidus: a late sequela of BNT162b2 SARS-CoV-2 mRNA vaccine? BMC Endocr Disord. 2023;23(1):47.
Partenope C, Pedranzini Q, Petri A, Rabbone I, Prodam F, Bellone S. AVP deficiency (central diabetes insipidus) following immunization with anti-COVID-19 BNT162b2 Comirnaty vaccine in adolescents: a case report. Front Endocrinol (Lausanne). 2023;14:1166953.
Matsuo T, Okubo K, Mifune H, Imao T. Bilateral Optic Neuritis and Hypophysitis with Diabetes Insipidus 1 Month after COVID-19 mRNA vaccine: Case Report and Literature Review. J Investig Med High Impact Case Rep. 2023;11:23247096231186046.
Johnston PC, Chew LS, Hamrahian AH, Kennedy L. Lymphocytic infundibulo-neurohypophysitis: a clinical overview. Endocr 2015, 50(3):531–6.
Sheen KC, Chang CC, Chang TC, Liu HM. Thickened pituitary stalk with central diabetes insipidus: report of three cases. J Formos Med Assoc. 2001;100(3):198–204.
Iwama S, Sugimura Y, Kiyota A, Kato T, Enomoto A, Suzuki H, Iwata N, Takeuchi S, Nakashima K, Takagi H, et al. Rabphilin-3A as a targeted autoantigen in lymphocytic infundibulo-neurohypophysitis. J Clin Endocrinol Metab. 2015;100(7):E946–954.
Di Iorgi N, Allegri AE, Napoli F, Calcagno A, Calandra E, Fratangeli N, Vannati M, Rossi A, Bagnasco F, Haupt R, et al. Central diabetes insipidus in children and young adults: etiological diagnosis and long-term outcome of idiopathic cases. J Clin Endocrinol Metab. 2014;99(4):1264–72.
Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101412.
Checinska Z, Galazka A, Smolik R. [A case of diabetes insipidus after smallpox vaccination]. Przegl Lek. 1966;22(6):454–5.
Palmar I, Kaljalovic R, Popovic M, Marcetic V. [Encephalopathy after vaccination against smallpox with permanent sequel–diabetes insipidus]. Vojnosanit Pregl. 1972;29(5):242–4.
Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480.
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. e278.
Chigr F, Merzouki M, Najimi M. Autonomic Brain centers and Pathophysiology of COVID-19. ACS Chem Neurosci. 2020;11(11):1520–2.
Ohashi A, Takeda Y, Watada M, Ihara F, Oshita T, Iwata N, Fujisawa H, Suzuki A, Sugimura Y, Maeda Y. Central diabetes insipidus with anti-rabphilin-3A antibody positivity causing hypovolemic shock after resection of tumorous lesions in the pelvic cavity. CEN Case Rep. 2023;12(3):297–303.
Acknowledgements
The authors express their gratitude to the patient and their family, who consented to the use of data and images for this case report.
Funding
This report received no funding support.
Author information
Authors and Affiliations
Contributions
All the authors contributed to authorship. H.T., H.G. and H.W. were involved in writing and drafting the case report. H.T., H.G., S.A. and T.U. were involved in the diagnosis and management of this patient. N.I., H.F., A.S. and Y.S. contributed to the measurement of anti-rabphilin-3A antibodies. All authors reviewed and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was not necessary for the reported investigations, as they were performed in a routine clinical setting and therapeutic intention. Written informed consent was obtained from the patient before undergoing all clinical procedures.
Informed consent
Written informed consent was obtained from the patient.
Consent for publication
Written informed consent was obtained from the parents of the study participants. A copy of the written consent is available for review by the Editor of this journal.
Disclosure
There are no COIs to disclose in this report.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Takizawa, H., Goto, H., Uchida, T. et al. Arginine vasopressin deficiency onset after COVID-19 vaccination with positive anti-rabphilin-3A antibodies: a case report and literature review. BMC Endocr Disord 24, 143 (2024). https://doi.org/10.1186/s12902-024-01664-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01664-8