Abstract
Background
The proportion of heart failure patients with preserved ejection fraction has been rising over the past decades and has coincided with increases in the prevalence of obesity and metabolic syndrome. The relationship between these interconnected comorbidities and heart failure with preserved ejection fraction (HFpEF) is still poorly understood. This study characterized obesity and metabolic syndrome among real-world patients with HFpEF.
Methods
We identified adults with heart failure in the Veradigm Cardiology Registry, previously the PINNACLE Registry, with a left ventricular ejection fraction measurement ≥ 50% between 01/01/2016 and 12/31/2019. Patients were stratified by obesity diagnosis and presence of metabolic syndrome (≥ 3 of the following: diabetes, hypertension, hyperlipidemia, and obesity). We captured baseline demographic and clinical characteristics and used multivariable logistic regression to examine the odds of having cardiac (atrial fibrillation, coronary artery disease, coronary artery bypass surgery, myocardial infarction, and stroke/transient ischemic attack) and non-cardiac (chronic kidney disease, chronic liver disease, and peripheral artery disease) comorbidities of interest. The models adjusted for age and sex, and the main covariates of interest were obesity and metabolic burden score (0–3 based on the presence of diabetes, hypertension, and hyperlipidemia). The models were run with and without an obesity*metabolic burden score interaction term.
Results
This study included 264,571 patients with HFpEF, of whom 55.7% had obesity, 52.5% had metabolic syndrome, 42.5% had both, and 34.3% had neither. After adjusting for age, sex, and burden of other metabolic syndrome-associated diagnoses, patients with HFpEF with obesity had lower odds of a diagnosis of other evaluated comorbidities relative to patients without obesity. The presence of metabolic syndrome in HFpEF appears to increase comorbidity burden as each additional metabolic syndrome-associated diagnosis was associated with higher odds of assessed comorbidities except atrial fibrillation.
Conclusion
Obesity was common among patients with HFpEF and not always co-occurring with metabolic syndrome. Multivariable analysis suggested that patients with obesity may develop HFpEF in the absence of other driving factors such as cardiovascular disease or metabolic syndrome.
Similar content being viewed by others
Background
Historically, heart failure (HF) has been associated with reduced ejection fraction (HFrEF); however, the incidence of heart failure with preserved ejection fraction (HFpEF) has been on the rise and may account for over half of newly diagnosed HF patients [1, 2]. In contrast to patients with HFrEF, HFpEF occurs more commonly in women and the elderly and is less responsive to most currently available medical therapy options, though some newer treatments show promise in this population [3,4,5,6].
Metabolic syndrome is a cluster of clinical measures, including increased waist circumference, elevated triglycerides, reduced high-density lipoprotein cholesterol, elevated blood pressure, and elevated fasting glucose, which are associated with an increased risk of cardiovascular disease [7]. Insulin resistance was initially thought to be the driver of metabolic syndrome [8]; however, newer research has suggested that metabolic syndrome derives from a complex interplay between obesity, hypertension, hyperlipidemia, and insulin resistance [9]. While abdominal obesity is one of the criteria for metabolic syndrome [7], some individuals exhibit metabolically healthy obesity in that they meet the body mass index (BMI) criteria for obesity in the absence of other metabolic comorbidities [10]. Despite both obesity and metabolic syndrome being risk factors for HFpEF [11,12,13], some evidence suggests that metabolically healthy obesity is not associated with an increased risk of HF [14, 15].
While obesity is prevalent among patients with HFpEF, there is conflicting evidence on whether it is a driver or bystander of HFpEF development and progression. Furthermore, we do not fully understand the clinical characteristics of these subpopulations. This cross-sectional analysis of the Veradigm Cardiology Registry sought to characterize profiles of patients with HFpEF stratified by obesity status, defined by BMI, and by metabolic syndrome status, defined by diagnosis codes, and characterize differences in patient profiles. We explored the interaction between obesity, metabolic syndrome, and the presence of other comorbidities in patients with HFpEF.
Methods
Data source
This retrospective analysis leveraged the Veradigm Cardiology Registry, previously the PINNACLE Registry. This registry was established in 2008 by the American College of Cardiology’s National Cardiovascular Data Registry to collect data on U.S. outpatient cardiovascular care of patients with HF, coronary artery disease, atrial fibrillation, or hypertension [16] and has been used in several studies of HF [17, 18]. Contributing clinical practices contribute longitudinal patient data, leveraging a technology platform to extract and standardize clinical data from electronic health records. Extracted data includes detailed information on symptoms, signs, medication prescribing, procedures, and outcomes [19, 20].
The dataset available for research contains only de-identified data as per the de-identification standard defined in Section § 164.514(a) of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy Rule. As a noninterventional, retrospective database study using data from a certified HIPAA–compliant de-identified research database, approval by an institutional review board was not required.
Study cohort
We identified adults, age 18 and older, in the Veradigm Cardiology Registry with at least one diagnosis of HF, at least one visit with a cardiologist, and a left ventricular ejection fraction (LVEF) measurement of ≥ 50% documented by a cardiologist between January 1, 2016, and December 31, 2019. The index date was the date of the first qualifying LVEF measurement. Patients were required to have a BMI within 365 days of the index date, non-missing gender on the index date, and no evidence of an LVEF measurement ≤ 40% within 365 days of the index date.
For the obesity analysis, patients were stratified by the presence or absence of a BMI ≥ 30, determined by the BMI value observed on the date closest to the index date, with ties going to dates occurring prior to the index date. Patients with obesity were further segmented by obesity class: class 1 (30 ≤ BMI < 35), class 2 (35 ≤ BMI < 40), and class 3 (BMI ≥ 40).
For the metabolic syndrome analysis, patients were coded as having metabolic syndrome if they had a prior diagnosis of at least three of the following four conditions: diabetes, hypertension, hyperlipidemia, and obesity.
Study variables
We captured age, sex (male or female), and race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, or other/unknown) on the index date. To comply with de-identification standards, patient age was truncated at 80 years old in the registry. In addition to the comorbidities used to define the study cohorts, we also captured prior diagnosis of atrial fibrillation, coronary artery disease, coronary artery bypass surgery, chronic kidney disease, chronic liver disease, myocardial infarction, peripheral artery disease, and stroke/transient ischemic attack (TIA) using the data fields defined in the Registry [20].
Where available, we also captured select laboratory results and vitals, including systolic blood pressure, diastolic blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, estimated glomerular filtration rate (eGFR), and New York Heart Association Functional Classification. For each measure, we took the value observed on the date closest to the index date, with ties going to dates occurring prior to the index date.
Data analysis
Categorical variables were reported as counts and percentages, while continuous variables were reported as medians and interquartile ranges (IQR).
We used multivariable logistic regression to help understand the relationship between obesity, metabolic syndrome, and the presence of other comorbidities in patients with HFpEF. We evaluated 8 independent generalized linear models, each using a logit link and binomial distribution to estimate the adjusted odds of patients having each comorbidity of interest. The comorbidities of interest were atrial fibrillation, coronary artery disease, coronary artery bypass surgery, chronic kidney disease, chronic liver disease, myocardial infarction, peripheral artery disease, and stroke/TIA.
Comorbid obesity was the independent variable of interest in all models. Models also adjusted for age (18–64, 65–79, and 80+), sex (male and female), and metabolic burden score (0–3). We defined the metabolic burden score as an ordinal variable with a value of 0, 1, 2, or 3 depending on the number of additional metabolic syndrome-associated diagnoses (diabetes, hypertension, and hyperlipidemia) identified in the patient record. Missing values were not imputed. To account for a possible interaction between the obesity and metabolic burden score, we ran all 8 models with and without an interaction term. Results of the logistic regression are reported as odds ratios with an associated 95% confidence interval (CI).
Data processing was conducted with SQL: ANSI (Dec 2021 release) within the Snowflake Data platform using Dbeaver Community version 22.2.0. The logistic regression was conducted in R version 4.2.2.
Results
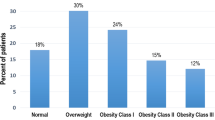
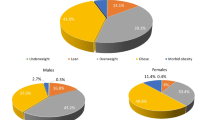
We identified 264,571 patients with HFpEF in the Veradigm Cardiology Registry that met the patient selection criteria (Fig. 1). The median (IQR) age of patients with HFpEF was 76 (67–80) years (Table 1). Overall, 53.1% were female, 59.6% were non-Hispanic White, and 7.8% were non-Hispanic Black. Among all patients with HFpEF, 55.7% had a BMI ≥ 30 (mean [SD]: 32.8 [8.3]), indicating obesity-related HFpEF, while 89.4% had a diagnosis of hypertension, 71.4% had a diagnosis of dyslipidemia, and 36.8% had a diagnosis of diabetes. Notably, 52.5% of the overall HFpEF population had metabolic syndrome, 42.5% had both obesity and metabolic syndrome, and 34.3% had neither obesity nor metabolic syndrome (Fig. 2).
Patients with obesity-related HFpEF were younger (median age: 72 years vs. 80 years), female (57.8% vs. 47.3%), and a higher percentage were non-Hispanic Black (9.8% vs. 5.2%) than patients with HFpEF without obesity (Table 1). Within this subgroup of patients with obesity-related HFpEF, the same relationships were maintained with obesity class. Higher obesity class correlated with younger age, a higher percentage of female individuals, and a higher percentage of non-Hispanic Black individuals (Table 2). In particular, the percentage of patients at least 80 years old decreased from 45.9% among patients with class 1 obesity to 19.6% among patients with class 3 obesity, and the median age decreased from 75 years to 68 years.
Among patients with obesity-related HFpEF, 90.9% had a diagnosis of hypertension, 71.5% had a diagnosis of dyslipidemia, and 43.8% had a diagnosis of diabetes (Table 1). When examined by obesity class, the prevalence of hypertension was similar across classes, ranging from 90.4 to 91.5% (Table 2). The prevalence of dyslipidemia decreased from 74.5% in class 1 obesity to 66.5% in class 3 obesity, whereas the prevalence of diabetes increased from 39.1% in class 1 obesity to 49.2% in class 3 obesity.
Among patients with HFpEF and metabolic syndrome, the most common contributing factors were hypertension (98.7%) and dyslipidemia (92.4%), while 64.6% had a diagnosis of diabetes and 81.1% had a BMI indicating obesity (Table 2). By comparison, among patients with HFpEF without metabolic syndrome, diabetes and obesity were present in only 6.2% and 27.7% of patient records, respectively.
In general, there appeared to be a negative correlation between obesity class and the prevalence of other cardiac conditions, such as coronary artery disease, atrial fibrillation, and stroke/TIA (Fig. 3a and Additional File 1). However, the prevalence of chronic kidney disease and chronic liver disease appeared to be independent of obesity class. Notably, with the exception of atrial fibrillation, the prevalence of both cardiac and non-cardiac conditions was higher in patients with HFpEF and metabolic syndrome compared to those with obesity-related HFpEF (Fig. 3b and Additional File 1).
Select vitals and laboratory results are reported in Additional File 1. Mean systolic and diastolic blood pressure ranged between 127.6–130.8 and 70.6–74.8, respectively, across the cohorts and were highest among HFpEF patients with class 3 obesity and lowest among HFpEF patients without obesity. Among the roughly one-third of patients with available labs, mean total cholesterol ranged from 153.2 to 159.2, mean HDL cholesterol ranged from 46.0 to 51.2, mean LDL cholesterol ranged from 79.9 to 85.7, and mean triglycerides ranged from 115.0 to 149.8. Among the 27.9% of patients with eGFR reported, all cohorts had a mean value of less than 60.
After adjusting for age, sex, and burden of other metabolic syndrome-associated diagnoses, patients with obesity-related HFpEF had lower odds of having a diagnosis of other evaluated comorbidities relative to patients with HFpEF and a BMI < 30 (Fig. 4). By contrast, each 1-point increase in metabolic burden score was associated with significantly higher odds of all comorbidities except atrial fibrillation. With two exceptions, older age, and male sex were associated with higher odds of comorbidities (Additional File 2).
Discussion
In this analysis of the Veradigm Cardiology Registry, obesity and metabolic syndrome were common among patients with HFpEF. The subset of patients with obesity-related HFpEF tended to be younger, female, and non-Hispanic Black compared to those without comorbid obesity. Patients with obesity-related HFpEF had lower odds of comorbidities than patients with HFpEF without obesity. By contrast, a higher metabolic burden score was associated with higher odds of all comorbidities except atrial fibrillation. This suggests patients with obesity may develop HFpEF in the absence of other driving factors, such as cardiovascular disease or metabolic syndrome.
In this study, coronary artery disease (CAD) was the comorbidity most strongly associated with higher metabolic burden in HFpEF. This is consistent with previous smaller clinical studies which have shown a higher prevalence of diabetes and dyslipidemia (as indicated by statin use) in HFpEF patients with comorbid CAD [21, 22]. Concerningly, these studies also found that CAD is also associated with poorer outcomes among patients with HFpEF [21, 22]. HFpEF with CAD is considered to be a distinct phenotype from HFpEF with obesity [23,24,25], but our analysis supports an overlap with other aspects of metabolic syndrome.
The majority of patients with obesity-related HFpEF in this study also met the criteria for metabolic syndrome. This is consistent with other studies, which have found a high degree of overlap between HF, obesity, and metabolic syndrome [26,27,28]. Obesity is an established risk factor for HF overall and HFpEF in particular [29, 30]; however, it is unclear whether it should be considered a risk factor independent of metabolic syndrome. While comorbid metabolic syndrome is associated with a higher risk of hospitalization for HF among patients with HFpEF [26], obesity may be protective against the development of HF in the absence of other metabolic factors [14, 15]. The findings of this study support exploring obesity as an independent driver of HFpEF rather than an outcome of cardiovascular comorbidities.
A comparative clinical assessment of patients with obesity-related HFpEF and those with non-obese HFpEF found significant differences in cardiac structure, function, and hemodynamics between cohorts [27]. Separately, among patients with HFpEF who appeared metabolically healthy, indicators of diastolic dysfunction were observed more frequently among individuals with higher BMI [31]. These findings, along with our own, suggest that efforts to identify subtypes of HFpEF may benefit from further refining the obesity-related HFpEF population by the presence of comorbid metabolic syndrome.
A review of observational studies of bariatric surgery among patients with obesity-related HF found that bariatric surgery was associated with improved quality of life, reduced readmission rates for HF, and improved New York Heart Association functional class [32]. While the studies included in the review article were not specific to HFpEF, a subsequent study found that bariatric surgery-induced body mass reduction was associated with improved functional scores, improved diastolic function, decreased left ventricular mass, and reduced resting heart rate in 12 women with obesity-related HFpEF [33]. This potential connection between body mass reduction and improved clinical outcomes for patients with obesity-related HFpEF has been tested in randomized controlled trials of the glucagon-like peptide 1 agonist and supported by the results of a recent study demonstrating that treatment with semaglutide 2.4 mg weekly led to larger reductions in symptomatic and physical burdens, greater improvements in functional capacity than placebo in patients with HFpEF and obesity [34]. Moreover, one randomized controlled trial of the glucagon-like peptide 1 receptor/glucose-dependent insulinotropic polypeptide receptor agonist, tirzepatide, is currently underway [35,36,37,38]. Based on the outcomes of these trials, further refinement may be necessary to determine if outcomes among patients with obesity-related HFpEF are contingent on the presence of comorbid metabolic syndrome.
Another prevailing theory is that obesity-associated HFpEF is driven by a comorbidity-induced systemic proinflammatory state [39,40,41]. For example, the microRNA miR-181c is differentially expressed in patients with diabetes and comorbid HFpEF, and overexpression of miR-181c has been associated with proinflammatory conditions [42]. Similarly, testosterone has demonstrated anti-inflammatory effects [43], and testosterone deficiency has been associated with HFpEF in males with a cardio-metabolic profile [44]. This proinflammatory state contributes to impaired autophagy of vascular smooth muscle cells and endothelial cells, increased interstitial fibrosis, and stiff cardiomyocytes [39, 45]. It is currently unclear whether targeting these comorbidity-induced inflammatory pathways could result in improved outcomes for patients with HfpEF, but it is an area of active research [46].
Another factor complicating the interpretation of this study is the interaction between age and BMI. Heart failure tends to be a disease of older adults; however, in this study, median patient age was lower among patients with obesity-related HFpEF. With the data available, we were unable to determine if this was due to earlier onset of HFpEF among patients with obesity or higher mortality among older adults with obesity, leading to a selection bias towards younger patients with obesity-related HFpEF. There are also questions about what is the appropriate BMI cut-off for older adults. For example, a recent analysis found that the optimal BMI for women over 65 may be above the cut-off of 30, commonly used to define obesity [47]. While BMI is a widely available metric in structured clinical records, new research suggests it may not be the ideal measurement for assessing health risks associated with excess body weight [48]; however, until there is a fundamental shift in routine data collection, BMI remains the most widely used metric for assessing obesity in retrospective observational research.
Limitations and strengths
This study is subject to several limitations inherent to the selected data source. First, the Veradigm Cardiology Registry was originally developed as a large outpatient quality improvement program based on voluntary participation and is, therefore, not a random sample of US cardiology practices. This may also impact the composition of patients included in the registry, as not all individuals with heart failure have access to specialty care. In addition, this data source should not be used to assess severe patient outcomes due to incomplete or lacking capture of key measures, such as hospitalizations and deaths.
Second, our study was not designed to identify an incident HFpEF population. Therefore, the documentation of a diagnosis should be interpreted as the patient had a diagnosis of the comorbidity of interest prior to the index date, not prior to the diagnosis of HFpEF. Laboratory results were only available for a subset of patients. Therefore, caution should be taken when assessing values overall and comparing values between cohorts, as there may be significant bias in which patients are being tested. For example, patients with suspected kidney disease may be more likely to have a documented eGFR value. As a result, laboratory results were not included as model covariates. Race and ethnicity were also not included as covariates due to the high degree of missingness.
Finally, we used BMI and diagnosis history as proxies for the standard clinical approach to assessing obesity and metabolic disease status [7]; however, these proxies are imprecise and may introduce bias into our analysis. Specifically, assessment for metabolic syndrome typically uses waist circumference or waist-to-hip ratio to identify at-risk individuals. The use of BMI as a proxy for obesity may overestimate the number of people with metabolically-relevant obesity and reduce the effect size of obesity as a predictor.
The strengths of this study include the size of the study population and the nature of the Veradigm Cardiology Registry. Specifically, patients in the registry are geographically distributed, sourced from small and large clinical practices, and come from a range of insurance plans, including Medicare, Medicaid, and private health plans [19]. In addition, the use of multivariable linear regression helps to detangle the contribution of obesity from overall metabolic burden while controlling for age and sex.
Conclusions
In this analysis of a large cardiovascular outpatient quality improvement registry, obesity was common among patients with HFpEF and was not always co-occurring with metabolic syndrome. In addition, we found that patients with obesity-related HFpEF had lower odds of other cardiac comorbidities, providing evidence that HF in this population can occur in the absence of other clinical burdens. Ongoing clinical trials will provide greater insight into the potential causal relationship between obesity and HFpEF.
Data availability
The data that support the findings of this study are available from Veradigm but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Veradigm.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- HF:
-
Heart failure
- HFpEF:
-
Heart failure preserved ejection fraction
- HFrEF:
-
Heart failure reduced ejection fraction
- HIPAA:
-
Health Insurance Portability and Accountability Act of 1996
- IQR:
-
Interquartile ranges
- LVEF:
-
Left ventricular ejection fraction
- TIA:
-
Transient ischemic attack
References
Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, et al. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham Study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging. 2018;11:1–11.
Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6:678–85.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61.
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20.
Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37:455–62.
Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28:1956–64.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94.
da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36:671–82.
Pujia R, Tarsitano MG, Arturi F, De Lorenzo A, Lenzi A, Pujia A, et al. Advances in phenotyping obesity and in its dietary and pharmacological treatment: a narrative review. Front Nutr. 2022;9:804719.
Liu L, Lima JAC, Post WS, Szklo M. Associations of time-varying obesity and metabolic syndrome with risk of incident heart failure and its subtypes: findings from the multi-ethnic study of atherosclerosis. Int J Cardiol. 2021;338:127–35.
Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6:701–9.
Clemenza F, Citarrella R, Patti A, Rizzo M. Obesity and HFpEF. J Clin Med. 2022;11:3858.
Yeh T-L, Chen H-H, Tsai S-Y, Lin C-Y, Liu S-J, Chien K-L. The relationship between metabolically healthy obesity and the risk of cardiovascular disease: a systematic review and meta-analysis. J Clin Med. 2019;8:1228.
Mirzababaei A, Djafarian K, Mozafari H, Shab-Bidar S. The long-term prognosis of heart diseases for different metabolic phenotypes: a systematic review and meta-analysis of prospective cohort studies. Endocrine. 2019;63:439–62.
Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (practice Innovation and Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14.
Ibrahim NE, Song Y, Cannon CP, Doros G, Russo P, Ponirakis A, et al. Heart failure with mid-range ejection fraction: characterization of patients from the PINNACLE Registry®. ESC Heart Fail. 2019;6:784–92.
Allen LA, Tang F, Jones P, Breeding T, Ponirakis A, Turner SJ. Signs, symptoms, and treatment patterns across serial ambulatory cardiology visits in patients with heart failure: insights from the NCDR PINNACLE® registry. BMC Cardiovasc Disord. 2018;18:80.
Maddox TM, Song Y, Allen J, Chan PS, Khan A, Lee JJ, et al. Trends in U.S. ambulatory cardiovascular care 2013 to 2017: JACC review topic of the week. J Am Coll Cardiol. 2020;75:93–112.
NCDR PINNACLE Registry. Data collection form v1.6. https://veradigm.com/img/pinnacle-data-collection-form.pdf. Accessed 13 Jan 2023.
Hwang S-J, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63 25PartA:2817–27.
Rusinaru D, Houpe D, Szymanski C, Lévy F, Maréchaux S, Tribouilloy C. Coronary artery disease and 10-year outcome after hospital admission for heart failure with preserved and with reduced ejection fraction. Eur J Heart Fail. 2014;16:967–76.
Samson R, Jaiswal A, Ennezat PV, Cassidy M, Le Jemtel TH. Clinical phenotypes in heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5:e002477.
Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation. 2016;134:73–90.
Anker SD, Usman MS, Anker MS, Butler J, Böhm M, Abraham WT, et al. Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension. Eur J Heart Fail. 2023;25:936–55.
Zhou Y, Fu L, Sun J, Zhu Z, Xing Z, Zhou S, et al. Association between metabolic syndrome and an increased risk of hospitalization for heart failure in population of HFpEF. Front Cardiovasc Med. 2021;8:698117.
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19.
Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602.
Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13.
Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res. 2023;118:3434–50.
Rozenbaum Z, Topilsky Y, Khoury S, Pereg D, Laufer-Perl M. Association of body mass index and diastolic function in metabolically healthy obese with preserved ejection fraction. Int J Cardiol. 2019;277:147–52.
Berger S, Meyre P, Blum S, Aeschbacher S, Ruegg M, Briel M, et al. Bariatric surgery among patients with heart failure: a systematic review and meta-analysis. Open Heart. 2018;5:e000910.
Mikhalkova D, Holman SR, Jiang H, Saghir M, Novak E, Coggan AR, et al. Bariatric surgery–induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity. 2018;26:284–90.
Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. New Engl J Med. 2023;389:1069–84.
Eli Lilly and Company. A randomized, double-blind, placebo-controlled, phase 3 study comparing the efficacy and safety of tirzepatide versus placebo in patients with heart failure with preserved ejection fraction and obesity (SUMMIT). Clinical trial registration: NCT04847557. https://clinicaltrials.gov/ct2/show/NCT04847557; 2023.
Novo Nordisk AS. Effect of semaglutide 2.4 mg once weekly on function and symptoms in subjects with obesity-related heart failure with preserved ejection fraction. Clin Trial Registration: NCT04788511. https://clinicaltrials.gov/ct2/show/NCT04788511; 2023.
Novo Nordisk AS. Effect of semaglutide 2.4 mg once-weekly on function and symptoms in subjects with obesity-related heart failure with preserved ejection fraction, and type 2 diabetes. Clinical trial registration: NCT04916470. https://clinicaltrials.gov/ct2/show/NCT04916470; 2023.
Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Christensen L, Davies M, et al. Design and baseline characteristics of step-hfpef program evaluating semaglutide in patients with obesity HFpEF phenotype. JACC Heart Fail. 2023;11(8 Pt 1):1000–10.
Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71.
Schiattarella GG, Rodolico D, Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc Res. 2021;117:423–34.
Dupas T, Pelé T, Dhot J, Burban M, Persello A, Aillerie V, et al. The endothelial dysfunction could be a cause of heart failure with preserved ejection fraction development in a rat model. Oxid Med Cell Longev. 2022;2022:7377877.
Jankauskas SS, Mone P, Avvisato R, Varzideh F, De Gennaro S, Salemme L, et al. miR-181c targets Parkin and SMAD7 in human cardiac fibroblasts: validation of differential microRNA expression in patients with diabetes and heart failure with preserved ejection fraction. Mech Ageing Dev. 2023;212:111818.
Bianchi VE. The anti-inflammatory effects of testosterone. J Endocr Soc. 2019;3:91–107.
Hamam A, Abou-Omar M, Rabah H, Khattab H, Alaarag A. Worsening effect of testosterone deficiency on males with heart failure with preserved ejection fraction. BMC Endocr Disord. 2022;22:321.
Sanhueza-Olivares F, Troncoso MF, Pino-de la Fuente F, Martinez-Bilbao J, Riquelme JA, Norambuena-Soto I, et al. A potential role of autophagy-mediated vascular senescence in the pathophysiology of HFpEF. Front Endocrinol (Lausanne). 2022;13:1057349.
Pugliese NR, Pellicori P, Filidei F, De Biase N, Maffia P, Guzik TJ, et al. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: implications for future interventions. Cardiovascular Res. 2022;118:3536–55.
Kıskaç M, Soysal P, Smith L, Capar E, Zorlu M. What is the optimal body Mass Index Range for older adults? Ann Geriatr Med Res. 2022;26:49–57.
Donini LM, Pinto A, Giusti AM, Lenzi A, Poggiogalle E. Obesity or BMI Paradox? Beneath the tip of the Iceberg. Front Nutr. 2020;7.
Acknowledgements
The authors would like to thank Stephanie Wall for her assistance with manuscript QA.
Funding
This work was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
JPB, LK, DRN, MB, and MM contributed to conceptualization, investigation, methodology, and interpretation of the data. KL contributed to investigation, methodology, data curation, formal analysis, and data validation. JPW contributed to investigation, visualization and developed the first draft of the manuscript. All authors contributed to critical review and editing of the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Veradigm Cardiology Registry only contains de-identified data as per the de-identification standard defined in Section § 164.514(a) of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy Rule. The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section § 164.514(b)(1) of the HIPAA Privacy Rule. U.S. law 45 Code of Federal Regulations Part 46, human participant protection regulations, and Protection of Human Subjects (45 CFR 46) requires institutional review board (IRB) approval for all research involving identifiable private information or identifiable biospecimens from human subjects as defined in 45 CFR 46.102(e)(5) and in 45 CFR 46.102(e)(6). However, protected health information that has been de-identified under the standard set for in 45 CFR 164.514(b)(1) is no longer considered to be identifiable health information and therefore is no longer subject to the IRB requirements set forth in 45 CFR 46. Because this study used only de-identified records, it is therefore no longer subject to the HIPAA Privacy Rule, the requirements for informed consent, or the IRB requirements set forth in 45 CFR 46. This study was conducted in compliance with the Declaration of Helsinki and used only de-identified data.
Consent for publication
Not Applicable.
Competing interests
LK, KL, JPW, and MB are employees of Veradigm, which received fees from Eli Lilly and Company related to this work. JPB, DRN, MM are employees of Eli Lilly and Company.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bae, J.P., Kallenbach, L., Nelson, D.R. et al. Obesity and metabolic syndrome in patients with heart failure with preserved ejection fraction: a cross-sectional analysis of the Veradigm Cardiology Registry. BMC Endocr Disord 24, 59 (2024). https://doi.org/10.1186/s12902-024-01589-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-024-01589-2