Abstract
Background
Several studies have highlighted the possible positive effects of soluble receptor for advanced glycation end products (sRAGE) against obesity. However, due to their inconsistent results, this systematic review and meta-analysis aimed to quantitatively evaluate and critically review the results of studies evaluating the relationship between sRAGE with obesity among adult population.
Methods
In the systematic search, the eligibility criteria were as follows: studies conducted with a cross-sectional design, included apparently healthy adults, adults with obesity, or obesity-related disorders, aged over 18 years, and evaluated the association between general or central obesity indices with sRAGE.
Results
Our systematic search in electronic databases, including PubMed, Scopus, and Embase up to 26 October, 2023 yielded a total of 21,612 articles. After removing duplicates, screening the titles and abstracts, and reading the full texts, 13 manuscripts were included in the final meta-analysis. According to our results, those at the highest category of circulating sRAGE concentration with median values of 934.92 pg/ml of sRAGE, had 1.9 kg/m2 lower body mass index (BMI) (WMD: -1.927; CI: -2.868, -0.986; P < 0.001) compared with those at the lowest category of sRAGE concentration with median values of 481.88 pg/ml. Also, being at the highest sRAGE category with the median values of 1302.3 pg/ml sRAGE, was accompanied with near 6 cm lower waist circumference (WC) (WMD: -5.602; CI: -8.820, -2.383; P < 0.001 with 86.4% heterogeneity of I2) compared with those at the lowest category of sRAGE concentration with median values of 500.525 pg/ml. Individuals with obesity had significantly lower circulating sRAGE concentrations (WMD: -135.105; CI: -256.491, -13.72; P = 0.029; with 79.5% heterogeneity of I2). According to the subgrouping and meta-regression results, country and baseline BMI were possible heterogeneity sources. According to Begg’s and Egger’s tests and funnel plots results, there was no publication bias.
Conclusion
According to our results, higher circulating sRAGE concentrations was associated with lower BMI and WC among apparently healthy adults. Further randomized clinical trials are warranted for possible identification of causal associations.
Similar content being viewed by others
Introduction
Advanced glycation end-products (AGEs) are a group of compounds formed by non-enzymatic glycation of lipids, proteins, and nucleic acids [1, 2]. AGEs have two endogenous and exogenous sources, while the first is formed during normal metabolism in body and the second is derived from foods or tobacco smoke [3]. AGEs are potential ingredients that lead to oxidative stress and chronic inflammation; they act through binding to their receptors like receptor for advances glycation end products (RAGE) [4]. RAGE is present in numerous differentiated adult cell types, including immune and endothelial, and is a multi-ligand receptor belonging to immunoglobulin superfamily [5,6,7]. AGE binding to its receptor, RAGE, triggers the cell signaling pathways’ activation through p38 and p44/42 MAP kinase, or nuclear factor kappa- B (NF-κB), and leads to production of reactive oxygen species (ROSs) and pro-inflammatory cytokines production [5, 8]. RAGE is expressed on numerous cell types, including vascular cells, adipocytes, podocytes, immune cells, neurons, cardio-myocytes, and lung epithelial cells [9,10,11,12]. RAGE has numerous ligands other than AGEs that can bind to either extracellular V-type immunoglobulin (Ig) domain with numerous special and distinct binding sites or to extracellular C1 and C2-type Ig domains; these further confirm the complexity of RAGE-ligand interactions [13,14,15].
Soluble receptor for advanced glycation end products (sRAGE) are a particular form of RAGE found in plasma and other fluids of the body, such as synovial fluid and cerebrospinal fluids [16]. There are two major forms of sRAGE; most of the circulating sRAGE results from cell surface-cleavage of the full-length receptor by matrix metalloproteinases (MMPs) [17, 18] and the other less prevalent form of sRAGE, known as endogenous secretory or esRAGE, is a product of a splice variant of AGE receptor (AGER) [19]. Numerous evidence are available about the pathogenic role of AGE and RAGE in inducing inflammation, oxidative stress, adipocyte hypertrophy and expansion, as well as ectopic lipid accumulation in different organs [20,21,22]. On the other hand, it is suggested that sRAGE, working as a decoy receptor, can bind to RAGE ligands and prevent membrane RAGE activation and associated detrimental health effects [23, 24]. Several studies have investigated the positive effects of sRAGE in prevention of obesogenic effects of AGE and its receptor RAGE. Dozio E et al. [24] reported lower sRAGE concentrations in women with obesity versus women with normal weight (P < 0.05). In another study by Zaki M et al. [25], similar finding was reported. Some other studies also reported positive associations between BMI and sRAGE concentrations [26,27,28,29]. However, some other studies reported no significant association between BMI and sRAGE [30, 31]. Since the exact association between obesity and sRAGE is not clear, a critical analysis can help to better identify this association. Accordingly, we aimed to quantify and critically review the results of studies reporting the associations between sRAGE with central or general obesity indices in general adult populations.
Methods and materials
To report the results, we used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Sup. Table 1) [32].
Eligibility criteria
In the current systematic review and meta-analysis, inclusion criteria were as follows: (1) observational studies with cross-sectional design (2); studies evaluating the relationship between sRAGE and obesity; (3) studies including measurements of general or central obesity including body mass index (BMI), fat mass, waist circumference (WC), or waist to hip ratio (WHR); (4) studies conducted among apparently healthy adults, with obesity or obesity-related disorders including diabetes, cardiovascular diseases, metabolic syndrome, and aged over 18 years; and (5) studies that provided the mean ± standard deviation (SD) of BMI, fat mass, WC, or WHR of those in the lowest versus highest categories of sRAGE. Interventional trials, case-reports and case-series, animal models and in vitro studies, reviews, congress, seminars, letter to editors and short communications were excluded. The PECO (patients, exposure, control/comparator and outcome) model for selecting the studies is presented in Table 1. This model is one of the most widely used models for formulating clinical questions.
Information sources, search strategy and selection process
Our systematic search in electronic databases, including Scopus, PubMed and Embase up to 26 October, 2023 yielded a total of 21,612 articles. The search was limited only to English language articles. No missing document was found through hand-searching the reference lists of the papers. The search strategy was created with a combination of the MeSH (Medical Subject Headings) (Sup. Table 2). Two independent reviewers evalutaed the articles for meeting the inclusion criteria. Any problem was resolved by a third reviewer in case of inconsistencies.
Data collection and extraction process
Four independent researchers performed data extraction, of the following information, including the name of first author, journal, country, publication year, demographic information of participants (e.g., age, gender distribution, and percent of male participants), baseline BMI and WC, study design, sample size, adjusted covariates, study setting, sRAGE measurement tools, and main findings.
Risk of bias assessment
The methodological quality of included studies for risk of bias assessment were assessed using the Agency for Healthcare Research and Quality (AHRQ) checklist [33, 34].
Synthesis methods
Data analysis was performed by STATA version 16 (STATA Corp, College Station, TX, USA). P-values less than 0.05 were considered as statistically significant. The studies that reported the comparison of BMI or WC [mean (SD)] in those with highest versus lowest sRAGE categories were evaluated. Therefore, the mean and SD of variable was used to calculate weighted mean difference (WMD) with 95% confidence interval (CI). When the median and range were reported and mean and SD were not available, the median values were considered as the best estimate of mean and the SD was calculated as the Hozo et al method as below: \({SD}^{2}\approx \left(\frac{1}{12}\right(\frac{{\left(a-2m+b\right)}^{2}}{4}+{\left(b-a\right)}^{2}\)) where “SD” is standard deviation, “a” and “b” are upper and lower limits of range, and “m” is the median value [35, 36]. For missing SDs, the method of Walter and Yao was used [37]. Cochran’s Q and I2 tests were used for heterogeneity measurements; for statistically significant heterogeneities (e.g., either P value for Q statistics of less than 0.1 or I2 greater than 50%), random effects model we used [38,39,40]. To identify the source of heterogeneity, subgrouping and meta-regression approaches were performed. Begg’s Funnel plots, Begg’s correlation coefficient and Egger’s asymmetry tests were used for assessment of publication bias.
Results
Study selection
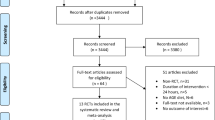
Our search results yielded a total of 21,612 articles that were imported into the EndNote software. In the first phase, 12,704 articles were removed due to duplication. Next, 8,192 articles were removed after screening the title and abstract due to not meeting inclusion criteria, other designs and age groups, and being seminars, congresses, and review articles. Finally, a total of 716 articles remained for full-text evaluation by two independent reserachers. Consequently, 13 manuscripts were included in the final meta-synthesis. The study’s selection flowchart is presented in Fig. 1.
Study characteristics
In the meta-analysis of the comparison of BMI between highest versus lowest sRAGE categories, six individual studies with a total number of 1,865 participants were included. General characteristics of these studies are shown in Table 2. Two out of eight studies were performed among obese, diabetic patients [31, 41] and the six other studies [25,26,27,28, 30, 42] were performed among apparently healthy population. The age range of study participants was 18–83 years old and BMI was 18–42 kg/m2. In the study by Momma H (2014) [26], BMI was significantly lower in the highest versus the lowest category of sRAGE among general Japanese adult population (22.7 versus 24.5 kg/m2; P < 0.001). Similarly, in another study by the same author [27], BMI was significantly lower in the highest versus the lowest category of esRAGE (21.9 versus 23.3 kg/m2; P < 0.001). Other studies by Moriya S et al. in general healthy population of Japan [28], by Moy KA et al. in Finland [30], and by Zaki M et al. in Egypt [25] found similar results. Similarly, in the meta-analysis of the comparison of WC between highest versus lowest sRAGE categories, four individual studies with 1,876 participants from the USA [42], Japan [26, 27], and Egypt [25], WC was significantly lower in the highest versus the lowest category of sRAGE in all the studies. The mean sRAGE concentration in serum was compared between individuals with obesity versus individuals without obesity in three individual studies with 165 participants [31, 41]; the individuals with obesity had lower mean sRAGE compared to individuals without obesity.
Risk of bias in the included studies
The results of risk of bias assessment is provided in Table 3 by AHRQ checklist [33]. As shown in this Table, four out of eight studies had moderate quality and the remaining four studies had high quality. The lowest quality score was 5 and the highest quality score was 9.
Results of synthesis
The results of meta-analysis (Fig. 2) showed that being at the highest category of sRAGE with median values of 934.92 pg/ml of sRAGE, was associated with lower BMI among apparently healthy adults (WMD: -1.927; CI: -2.868, -0.986; P < 0.001) compared with those at the lowest category of sRAGE concentration with median values of 481.88 pg/ml. Similarly, those at the highest sRAGE category with the median values of 1302.3 pg/ml sRAGE had about 6 cm lower WC compared with those at the lowest category of sRAGE concentration with median values of 500.525 pg/ml (WMD: -5.602; CI: -8.820, -2.383; P < 0.001; Fig. 3). Comparing the sRAGE concentrations in individuals with obesity versus individuals without obesity, individuals with obesity had significantly lower circulating sRAGE concentrations (WMD: -135.105; CI: -256.491, -13.72; P = 0.029; Fig. 4). Subgrouping results of the comparison of BMI between the highest versus the lowest category of sRAGE are presented in Table 4. In studies performed in Japan that had baseline BMI of lower than 23 kg/m2, initial heterogeneity reduced from 86.4 to 0. Therefore, country and baseline BMI might be the sources of heterogeneity. The results of subgrouping for the comparison of WC between sRAGE categories are shown in Table 5. Accordingly, country might be a heterogeneity source. However, interpretation of results is challenging due to the low number of studies in each subgroup. The results of meta-regression (Tables 6 and 7) also confirmed these findings. The results of funnel plots (Sup. Figures 1, 2) and Begg’s adjusted rank correlation and Egger’s regression asymmetry tests showed no publication bias (for BMI; Egger P-value: 0.224; Begg’s P-value: 0.851 and for WC: Egger P-value: 0.497; Begg’s P-value: 0.297).
Discussion
In the current meta-analysis, higher sRAGE concentrations was associated with lower BMI among 1,865 apparently healthy individuals. Also, lower WC was accompanied with higher sRAGE concentrations among 1,876 adults. This is the first meta-analysis evaluating the association between sRAGE and obesity indices, and reporting obesity-reducing effects of sRAGE among adults. There are several underlying mechanisms suggested for obesity prevention by sRAGE, such as protection against obesity-induced lipid accumulation by preventing RAGE hyper-expression [43]. Also, it is suggested that higher AGE flux in individuals with obesity reduces sRAGE levels; it is well-known that sRAGE has other ligands besides AGEs that could decrease it. So, a lower sRAGE among individuals with obesity is a reflection of greater binding of AGEs to its ligands [44, 45]. Since numerous studies revealed lower circulating AGEs concentrations in individuals with obesity, one interesting finding is that, possibly, CML concentrations are not a good biomarker of its status in individuals with obesity, and further studies are needed to confirm whether it is the case for different AGE compounds [46,47,48,49]. This might be attributed to the fact that the most preferable AGEs measured in the studies is CML, and it is suggested that circulating CML concentrations is in inverse association with body fat storage, because it deposits in fat tissue and by fat mass expansion (like in individuals with obesity) its deposition in fat mass increases and lowers its circulating amount [48, 50, 51]. As a result, circulating sRAGE values would be a better marker of the AGE-RAGE interaction in the body and its lower concentrations is a reflection of detrimental effects of this interaction. Impaired adipocyte function is also suggested as possible mechanism of increased central obesity in lower sRAGE concentrations [26, 52]. The possible underlying mechanisms of the effects of AGE-RAGE interaction in developing obesity and the protective role of sRAGE is presented in Fig. 5.
The accumulation of AGEs in adipocytes may explain the inverse association between AGE levels and BMI; high-AGE diets are associated with greater fat consumption and an increased risk of abdominal obesity [55]. RAGE activation also raises circulating inflammatory cytokines while decreasing anti-inflammatory adipokines like adiponectin. IL-6 and leptin are recognized as inflammatory mediators in the presence of obesity [56]. Moreover, adipocyte hypertrophy is associated with enhanced RAGE expression [57]. However, the involvement of sRAGE in the prevention of age-related muscle mass loss must be addressed [58]. As a decoy receptor for AGEs and other inflammatory ligands, sRAGE attributed to the reduced ligand-mediated disruption, preventing AGEs from binding to the cell-bound full-length receptor RAGE [58] and inhibiting the obesogenic effects of AGE-RAGE interaction by diminishing AGES accumulation visceral fat, regulating leptin and Adiponectin release [56], reducing Insulin resistance [58]. AGEs = advanced glycation end products; RAGE = receptor for advanced glycation end products; sRAGE = soluble receptor for advanced glycation end products; IL-1 = interleukin-1; IL-6 = interleukin-6; TNF-α = Tumor Necrosis Factor alpha; BMI = Body mass index; WC = Waist Circumference
In our study, all the included studies had a moderate or high quality, and no study had poor quality. In the subgrouping, geographical area and baseline BMI were the possible sources of heterogeneity. However, the current meta-analysis has some limitations. First, causal inference was not possible due to cross-sectional design of the included studies. Second, due to the low number of studies in each subgroup, making a reliable conclusion is not possible. Third, as the adipose tissue differs between men and women [53, 54], we needed separate data in this regard; however, there was no separate data to evaluate the association of sRAGE levels with BMI or WC by gender.
As a conclusion, in the current systematic review and meta-analysis, for the first time, we quantified and critically reviewed the studies that evaluated the association between general or central obesity indices with circulating sRAGE levels. There was a negative association between BMI, WC, and circulating sRAGE concentrations among adults. Further studies are warranted to confirm our results.
Data Availability
The datasets generated and/or analyzed in the current study are not publicly available due to some restrictions applied by the ethics committee. However, they are available from the corresponding author on reasonable request.
References
Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46.
Hjazi A. The effects of Capsicum annuum supplementation on lipid profiles in adults with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Phytoth Res. 2023;37(9):3859–66.
Abe R, Yamagishi S. AGE-RAGE system and carcinogenesis. Curr Pharm Des. 2008;14:940–5.
Cheng C, Tsuneyama K, Kominami R, Shinohara H, Sakurai S. Expression profiling of endogenous secretory receptor for advanced glycation end products in human organs. Mod Pathol. 2005;18:1385–96.
Riehl A, Nemeth J, Angel P, Hess J. The receptor RAGE: bridging inflammation and cancer. Cell Commun Signal. 2009;7:12–5.
Gallegos-Gonzalez G, Pineda-García G, Serrano-Medina A, Martinez AL, Ochoa-Ruiz E. Association between stress and metabolic syndrome and its mediating factors in university students. Ame J Health Behav. 2021;45(6):1091–102.
Mahamda HA, Al Alwany AA. Influence of Syphilis Infection on abortions in Iraq. J Communic Dis. 2022;54(4):41–5.
Bierhaus A, Nawroth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and Diabetes Mellitus and its Complications. Diabetologia. 2009;52:2251–63.
Shekhtman A, Ramasamy R, Schmidt AM. Glycation and the RAGE axis: targeting signal transduction through DIAPH1. Expert Rev Proteom. 2017;14(2):147–56.
Ramasamy R, Shekhtman A, Schmidt AM. The multiple faces of RAGE–opportunities for therapeutic intervention in aging and chronic Disease. Expert Opin Ther Targets. 2016;20(4):431–46.
Al Alwany AA. Arrhythmia related to hypertensive left ventricular hypertrophy in Iraqi patients: frequency and outcome. J Med Life. 2022;15(9):1115–8.
Al Alwany AA. Echocardiographic assessment of the aortic stenosis valve area: parameters and outcome. Echocardiography. 2022;5(7):1281–8.
Egaña-Gorroño L, López-Díez R, Yepuri G, Ramirez LS, Reverdatto S, Gugger PF, Shekhtman A, Ramasamy R, Schmidt AM. Receptor for advanced glycation end products (RAGE) and mechanisms and therapeutic opportunities in Diabetes and Cardiovascular Disease: insights from human subjects and animal models. Front Cardiovas Med. 2020;7(37):1–16.
Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim et Biophys Acta (BBA)-Mol Cell Res. 2009;1793(6):993–1007.
Kamolthip R, Fung XCC, Lin CY, Latner JD, O’Brien KS. Relationships among physical activity, health-related quality of life, and weight stigma in children in Hong Kong. Am J Health Behav. 2021;45(5):828–42.
Schmidt AM. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory Disease. Vascular Pharmacol. 2015;72:1–8.
Zhang L, Bukulin M, Kojro E, Roth A, Metz VV, Fahrenholz F, Nawroth PP, Bierhaus A. Postina R. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biolog Chem. 2008;283(51):35507–16.
Metz VV, Kojro E, Rat D, Postina R. Induction of RAGE shedding by activation of G protein-coupled receptors. PLoS ONE. 2012;2012(7):e41823.
Yonekura H, Yamamoto Y, Sakurai S, Watanabe T, Yamamoto H. Roles of the receptor for advanced glycation endproducts in diabetes-induced vascular injury. J Pharmacol Sciences. 2005;97(3):305–11.
Gaens KH, Stehouwer CD, Schalkwijk CG. Advanced glycation endproducts and its receptor for advanced glycation endproducts in obesity. Curr Opin Lipidol. 2013;24(1):4–11.
Dozio E, Vianello E, Briganti S, Lamont J, Tacchini L, Schmitz G, Corsi Romanelli MM. Expression of the receptor for advanced glycation end products in epicardial fat: link with tissue thickness and local insulin resistance in coronary artery disease. J Diab Res, 2016. 2016: 2327341.
Leung C, Herath CB, Jia Z, Goodwin M, Mak KY, Watt MJ, Forbes JM. Angus PW. Dietary glycotoxins exacerbate progression of experimental fatty Liver Disease. J Hepatol. 2014;60(4):832–8.
Geroldi D, Falcone C, Emanuele E. Soluble receptor for advanced glycation end products: from Disease marker to potential therapeutic target. Curr Med Chem. 2006;13(17):1971–8.
Dozio E, Briganti S, Delnevo A, Vianello E, Ermetici F, Secchi F, Sardanelli F, Morricone L, Malavazos AE, Corsi Romanelli MM. Relationship between soluble receptor for advanced glycation end products (sRAGE), body composition and fat distribution in healthy women. Eur J Nutr. 2017;56(8):2557–64.
Zaki M, Kamal S, Kholousi S, El-Bassyouni HT, Yousef W, Reyad H, Mohamed R, Basha WA. Serum soluble receptor of advanced glycation end products and risk of metabolic syndrome in Egyptian obese women. Excli j. 2017;16:973–80.
Momma H, Niu K, Kobayashi Y, Huang C, Chujo M, Otomo A, Tadaura H, Miyata T, Nagatomi R. Higher serum soluble receptor for advanced glycation end product levels and lower prevalence of metabolic syndrome among Japanese adult men: a cross-sectional study. Diabetol Metab Syndr. 2014;6(1):33.
Momma H, Niu K, Kobayashi Y, Huang C, Chujo M, Otomo A, Tadaura H, Miyata T, Nagatomi R. Lower serum endogenous secretory receptor for advanced glycation end product level as a risk factor of metabolic syndrome among Japanese adult men: a 2-year longitudinal study. J Clin Endocrinol Metab. 2014;99(2):587–93.
Moriya S, Yamazaki M, Murakami H, Maruyama K, Uchiyama S. Two soluble isoforms of receptors for advanced glycation end products (RAGE) in carotid Atherosclerosis: the difference of soluble and endogenous secretory RAGE. J Stroke Cerebrovasc Dis. 2014;23(10):2540–6.
Al Alwany AA. Effect and benefit of percutaneous coronary intervention in chronic total occlusion on ventricular repolarization: QT correction and dispersion. J Med Life. 2022;15(8):1025.
Moy KA, Jiao L, Freedman ND, Weinstein SJ, Sinha R, Virtamo J, Albanes D, Stolzenberg-Solomon RZ. Soluble receptor for advanced glycation end products and risk of Liver cancer. Hepatology. 2013;57(6):2338–45.
Davis KE, Prasad C, Vijayagopal P, Juma S, Imrhan V. Serum soluble receptor for advanced glycation end products correlates inversely with measures of adiposity in young adults. Nutr Res. 2014;34(6):478–85.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61:1600324.
Guerra ZC, Moore JR, Londoño T, Castro Y. Associations of acculturation and gender with obesity and physical activity among latinos. Am J Health Behav. 2022;46(3):324–36.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;14(135):1–13.
Al-Alwany A. Latrogenic atrial septal defect post radiofrequency ablation in patients with left atrial SVT: predictors and outcomes. Revista Latinoam De Hipertensión. 2021;16(3):185–91.
Walter S, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol. 2007;60(8):849–52.
Higgins JP. Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549.
Chen S, Zhou Z, Ren K. Influence of sports value on adolescent participation and preference of Sci-Tech experience activities. Revista De Psicología Del Deporte. 2021;30(4):1.
Amin MN, Mosa AA, El-Shishtawy MM. Clinical study of advanced glycation end products in Egyptian diabetic obese and non-obese patients. Int J Biomed Sci. 2011;7(3):191–200.
Chen L, Duan Z, Tinker L, Sangi-Haghpeykar H, Strickler H, Ho GY, Gunter MJ, Rohan T, Logsdon C, White DL, Royse K, El-Serag HB., Jiao, L. A prospective study of soluble receptor for advanced glycation end-products and Colorectal cancer risk in postmenopausal women. Cancer Epidemiol. 2016;42:115–23.
Dozio E, Vianello E, Bandera F, Longhi E, Brizzola S, Nebuloni M, Corsi Romanelli MM. Soluble receptor for Advanced Glycation End products: a protective molecule against Intramyocardial lipid Accumulation in obese Zucker rats? Mediat Inflamm. 2019;2019:2712376.
Ramasamy R, Yan SF, Schmidt AM. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the Complications of Diabetes. Vascul Pharmacol. 2012;57(5–6):160–7.
Saleh AY, Mohammed AH, Al-Mashta WKAH, Al-Ameri MM, Al-Haili T, Alwan NH, Ateb FH. Moderating effect of physical health impairments between physical activity and sportsman learning experiences: mediating role of quality of life and physical self-esteem. RIMCAFD. 2022;22:60–77.
Rodríguez-Mortera R, Luevano-Contreras C, Solorio-Meza S, Gómez-Ojeda A, Caccavello R, Bains Y, Gugliucci A, Garay-Sevilla ME. Soluble receptor for Advanced Glycation End products and its correlation with vascular damage in adolescents with obesity. Horm Res Paediatr. 2019;92(1):28–35.
Garay-Sevilla ME, Torres-Graciano S, Villegas-Rodríguez ME, Rivera-Cisneros AE, Wrobel K, Uribarri J. Advanced glycation end products and their receptors did not show any association with body mass parameters in metabolically healthy adolescents. Acta Paediatr. 2018;107(12):2146–51.
Semba RD, Arab L, Sun K, Nicklett EJ, Ferrucci L. Fat mass is inversely associated with serum carboxymethyl-lysine, an advanced glycation end product, in adults. J Nutr. 2011;141(9):1726–30.
Mahmoudinezhad M, Farhangi MA, Kahroba H, Dehghan P. Personalized diet study of dietary advanced glycation end products (AGEs) and fatty acid desaturase 2 (FADS(2)) genotypes in obesity. Sci Rep. 2021;11(1):19725.
Schmidt AM, Stern D. Atherosclerosis and Diabetes: the RAGE connection. Curr Atheroscler Rep. 2000;2(5):430–6.
Fu J, Li L, Jiang K, Wang W. Impact of safety managment on mental health and quality of life in perioperative care of fitness players undergoing cardiac intervention. RIMCAFD. 2022;22(881):78–88.
Jawoosh HN, Fadhil AA, Rubaye A, Kadhim AWAL, Alghurabi AJ, Ahmed AMK, Elwan HM. MH. Health awareness mediation role amongst health card live story, health communication, health awareness programs, and health living culture: A study on Iraq medical colleges RIMCAFD, 2022. 22(88.1).
Hyder KM, Mohan J, Varma V, Sivasankaran P, Raja D. Effects of muscle–specific exercises compared to existing interventions on insulin resistance among Prediabetes Population of South India. J Nat Sci Biology Med. 2021;12(2):230.
de Haro VM, Peral-Rodríguez P, Cid-Yagüe L, Álvarez-Barrio M. Nueva forma de entender las pruebas de condición física en relación con la salud. Volume 22. Revista Internacional de Medicina y Ciencias de la Actividad Física y del Deporte; 2022. pp. 129–51. 85.
Paradela-Dobarro B, Fernandez-Trasancos A, Bou-Teen D, Eiras S, Gonzalez-Ferreiro R, Agra RM, Varela-Roman A, Castro-Pais AI, Carreira MC, Casanueva FF. Evolution and bad prognostic value of advanced glycation end products after acute Heart Failure: relation with body composition. Cardiovas Diabetol. 2017;16(1):1–11.
Miranda ER, Fuller KN, Perkins RK, Kroeger CM, Trepanowski JF, Varady KA, Haus JM. Endogenous secretory RAGE increases with improvements in body composition and is associated with markers of adipocyte health. Nutr Metab Cardiovasc Dis. 2018;28(11):1155–65.
Ribeiro PVM, Tavares JF, Costa MAC, Mattar JB, Alfenas RCG. Effect of reducing dietary advanced glycation end products on obesity-associated Complications: a systematic review. Nutr Rev. 2019;77(10):725–34.
Kim TN, Park MS, Lee EJ, Chung HS, Yoo HJ, Kang HJ, Song W, Baik SH, Choi KM. The association of low muscle mass with soluble receptor for advanced glycation end products (sRAGE): the Korean sarcopenic obesity study (KSOS). Diab Metab Res Rev. 2018;34(3):e2974.
Acknowledgements
This work was supported by Al-Mustaqbal University (Grant number: MUC-E-0122).
Funding
None.
Author information
Authors and Affiliations
Contributions
NAT wrote the first draft of manuscript, PR supervised the project and analyzed data, SHA and RM were extracted data and was involved in data analysis, SGA, AKK and RSZ were involved in quality assessment of studies, AMS, SHJ and YFM were involved in data extraction, analysis and English edition of the paper. All of the authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was registered in the International Prospective Register of Systematic Reviews system (PROSPERO). .
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Sup. Table 1
. PRISMA Checklist. Sup. Table 2. Search strategies and the number of records according to different electronic database. Sup. Figure 1. Begg’s funnel plot (with pseudo 95% CIs) of the weighted mean difference (WMD) versus the standard error (se) of (WMD) for the comparison of (A) body mass index (BMI), (B) waist circumference (WC) in those of the highest versus lowest soluble receptor for advanced glycation end products (sRAGE) categories [BMI: P egger= 0.224; P begg =0.851; WC, P egger= 0.297; P begg =0.497]
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tayyib, N.A., Ramaiah, P., Alshahrani, S.H. et al. Soluble receptor for advanced glycation end products (sRAGE) is associated with obesity rates: a systematic review and meta-analysis of cross-sectional study. BMC Endocr Disord 23, 275 (2023). https://doi.org/10.1186/s12902-023-01520-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01520-1