Abstract
Background
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor that originates from parafollicular C-cells. Calcitonin (Ctn) and carcinoembryonic antigen (CEA) are useful biomarkers for monitoring MTC cases.
Case presentation
Here, we describe a 48-year-old woman, who presented in 2014 with bilateral thyroid nodules. Report of fine needle aspiration was suspicious for MTC; initial laboratory evaluation showed serum Ctn level of 1567 pg/mL. After excluding type 2 multiple endocrine neoplasia syndrome clinically, total thyroidectomy and neck lymph node dissection were performed. The final histopathological diagnosis was right lobe MTC with neither vascular invasion nor lymph node involvement. On regular follow-up visits, Ctn and CEA levels have been undetectable, and repeated cervical ultrasonographic exams were unremarkable from 2014 to 2021. As liver enzymes became elevated in 2016, the patient was further evaluated by a gastroenterologist. Abdominopelvic ultrasonography revealed a coarse echo pattern of the liver parenchyma with normal bile ducts. A liver fibroscan showed a low fibrosis score (7kPa). The patient was recommended to use ursodeoxycholic acid. According to the progressive rise of liver enzymes with a cholestatic pattern in October 2020, a liver biopsy was performed that showed tiny nests of neuroendocrine-like cells with a background of primary biliary cholangitis (PBC). Immunohistochemical stainings were positive for chromogranin A (CgA), and synaptophysin and negative for Ctn, CEA, and thyroglobulin. Further imaging investigations did not reveal any site of a neuroendocrine tumor in the body. Considering normal physical exam, imaging findings, as well as normal serum levels of Ctn, CEA, CgA, and procalcitonin, the patient was managed as a PBC.
Conclusion
In follow-up of a patient with MTC, we reported progressively increased liver enzymes with a cholestatic pattern. Liver biopsy revealed nests of neuroendocrine-like cells with a background of PBC, the findings that might suggest acquiring neuroendocrine phenotype by proliferating cholangiocytes.
Similar content being viewed by others
Background
Medullary thyroid carcinoma (MTC) is a rare differentiated neuroendocrine tumor (NET), that originates from thyroid parafollicular C-cells, with an occurrence rate of 2–5% among all thyroid cancers [1]. C-cells of the thyroid gland produce several hormones and biogenic amines [2]. Calcitonin (Ctn) and carcinoembryonic antigen (CEA) are well-recognized biomarkers secreted from neoplastic C-cells in well-differentiated MTC. Considering the high sensitivity and specificity of these biomarkers, they are applied for monitoring the remission versus disease progression in individuals with MTC [2,3,4].
According to the revised American thyroid association (ATA) guideline, thyroid nodules suspected for or diagnosed as MTC in cytological or histopathological examinations, respectively, should be managed with neck ultrasound examination, determinations of serum Ctn and CEA levels, and DNA analysis for the rearranged during Transfection (RET) germline mutation [5]. In sporadic MTC, after total thyroidectomy and central neck lymph node dissection, follow-up by physical examination, neck ultrasonography, serum Ctn, and CEA levels are recommended [5]. Although, metastatic cases of MTC with normal serum levels of biomarkers were reported [2, 6], generally undetectable serum Ctn and CEA levels, in combination with a negative residual tumor on imaging examinations, exclude the presence of metastatic MTC [7].
In our case, a MTC with clinical and paraclinical remission, gradually rising liver enzymes with the presence of neuroendocrine-like cells in the liver biopsy could be explained by changes in the phenotype of cholangiocytes to neuroendocrine-like cells.
Case presentation
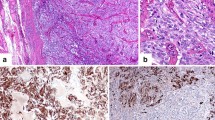
A 48-year-old woman, with no personal or family history of thyroid malignancy, was consulted for thyroid nodules in November 2014. Physical examination revealed multiple nodules in both thyroid lobes, without compression effects on adjacent organs. Ultrasonography of the neck documented one hypoechoic nodule 44 × 28 mm with microcalcification in the right lobe and two 14 × 8 mm and 9 × 6 mm isoechoic nodules in the left lobe with no neck lymphadenopathy. Fine needle aspiration cytology of the right lobe nodule was reported as suspicious for MTC and serum Ctn level was 1567 pg/mL (reference value: < 10 pg/mL). The patient was clinically and biochemically euthyroid. Her blood pressure was 110/70 mmHg. She did not complain of paroxysmal hypertension, palpitation, or sweating; also, she did not have personal or family history of renal stone or hypercalcemia. Laboratory tests including serum calcium, parathyroid hormone, and 24-h urine collection for vanillylmandelic acid, norepinephrine, and epinephrine were unremarkable (Table 1). The patient was scheduled for total thyroidectomy and bilateral cervical lymph node dissection in November 2014. Histopathological examination confirmed the diagnosis of right lobe MTC without vascular invasion, extracapsular extension, lymph node involvement, or C-cell hyperplasia. Immunohistochemical (IHC) stainings were positive for Ctn, synaptophysin, chromogranin A (CgA), and negative for thyroglobulin (Tg). RET mutation in the patient’s peripheral blood lymphocytes was positive in exon 11, codon 691, and exon 15, codon 904. Two months after thyroidectomy, serum Ctn and CEA levels were 1.3 pg/mL (reference value: < 10 pg/mL) and 4.9 ng/mL (reference value: < 2.5 ng/mL), respectively; with mildly elevated liver enzymes i.e., aspartate aminotransferase: 44 IU/L, alanine aminotransferase: 38 IU/L, and alkaline phosphatase (ALP): 380 IU/L. Since the calcium-loaded calcitonin test was not available at that time and considering the immediate post-operative significant decrement of serum calcitonin level, this test was not performed. On regular follow-up visits, serum Ctn and CEA levels were consistently below the upper limit of reference ranges from 2014 to 2021 (Fig. 1), and repeated cervical ultrasonography did not show abnormal findings. According to gradually raised liver enzymes with a cholestatic pattern (Fig. 2), the patient was referred to a gastroenterologist for evaluation of liver function in 2016. Abdominopelvic ultrasonography revealed a coarse echo pattern of the liver with normal internal and external bile ducts. Liver fibroScan showeda low fibrosis score (7kPa). Anti-mitochondrial antibody was 1.2 units (reference value: < 1.5 units), and other immunological tests and hepatitis serology panels were normal. Treatment with ursodeoxycholic acid 300 mg daily was initiated and the patient followed clinically and biochemically. Since liver enzymes especially ALP and gamma glutamyl transferase (GGT) gradually elevated in 2020, a liver biopsy was performed. The histopathological examination of the liver biopsy, reported by two experienced pathologists, showed tiny nests of neuroendocrine-like cells, severe portal lymphoplasma cell infiltration, some piecemeal necrosis, mild bile duct proliferation, focal parenchymal destruction of interlobular bile ducts by granulomatous reaction, and fibrous expansion of portal tracts with occasional p-p bridges (Fig. 3). IHC staining showed diffuse mild to moderate positivity for synaptophysin. IHC staining for CgA was positive in 80% of cells with moderate intensity (Fig. 4). Negative results were reported for Ctn, CEA, and Tg. The proliferation marker of Ki67 was positive in 2–3% of the neuroendocrine-like cells. Histopathologic findings and IHC stainings were compatible with the presence of neuroendocrine-like cells in a background of primary biliary cholangitis (PBC). Results of upper and lower gastrointestinal endoscopies, whole body bone scan with TC99 and 68Ga-Dotatate positron emission tomography (PET)-CT scan were unremarkable. Abdominal computed tomography (CT) showed few periportal and portocaval lymph nodes (up to 10 × 14 mm). Endoscopic ultrasound evaluation only suggested a few hilar and peripancreatic lymph nodes. Subsequent FNA of hilar lymph node was negative for NET or any other malignancy, and IHC stainings were negative for cytokeratin, synaptophysin, Ctn, and CgA. Serum CgA and procalcitonin levels were reported 43 ng/mL (reference value: < 100 ng/mL) and 0.1 ng/mL (reference value: < 0.15 ng/mL), respectively. Hence, according to negative biochemical and radiological evidence for primary or secondary hepatic NETs, as well as the presence of obstructive liver pathology, the final diagnosis of anti-mitochondrial autoantibodies (AMA)-negative PBC was made and the patient was managed with glucocorticoid.
Histopathologic sections of the liver biopsy (×100 and × 400 magnifications): A and B, Liver tissue with cholestatic pattern of injury and bile duct damage (H&E stain); C and D, Fibrous expansion of portal tract with occasional p-p bridges (Trichrome stain); E and F Nests of neuroendocrine-like cells (H&E stain)
Discussion and conclusions
Here, we reported a case of MTC with high serum Ctn level and no neck lymph node involvement at presentation. After total thyroidectomy, the patient had been in a complete biochemical and clinical remission, with undetectable serum biomarkers and normal neck sonography through 6 years. During follow-up, a gradual rising of liver enzymes with a cholestatic pattern was seen. Histopathological evaluation of liver biopsy revealed tiny nests of neuroendocrine-like cells in a background predominantly with a cholestatic pattern, in favor of PBC, although imaging findings indicated no evidence of metastasis.
Three potential scenarios could be proposed for our case. Considering the patient’s history of MTC, the most probable explanation for the presence of nests of neuroendocrine-like cells in the liver biopsy was late metastatic MTC to the liver with low serum Ctn level. MTC, an uncommon and aggressive thyroid cancer, could occur sporadically or hereditary as a component of type 2 multiple endocrine neoplasia syndrome [6]. Regarding the sequence of the coding region of RET proto-oncogene mutation in this case that was not in moderate or high-risk categories, as well as considering the occurrence of somatic RET mutation in 50% of sporadic MTCs [5], we considered this patient as a sporadic case of MTC and followed her with repeated cervical neck ultrasonographic exams and serum Ctn and CEA levels in approximately 6–12 month intervals. C-cells of the thyroid gland secrete several hormones and biogenic amines [5], Ctn and CEA are two useful tumor markers secreted from these cells in MTC. Although these biomarkers could be increased in different conditions [5], their monitoring is helpful in postoperative follow-up and determining the prognosis of individuals with MTC [7]. In rare cases of metastatic MTC, serum Ctn and CEA levels could be normal or undetectable. Several reasons have been advocated to explain this medical dilemma. The hook effect is one of the possible explanations; however, in our case, we did not recheck the Ctn level in serially diluted samples. Some authors hypothesize dedifferentiation as a leading cause of the inability of a tumor to produce Ctn [2]. Studies reported various behavior of MTC regardless of the initial stage and the presence or absence of distant metastasis ranging from prolonged indolent course to highly aggressive one [8]. Recently, Park et al. evaluate the clinical characteristics and long-term oncologic outcomes of 46 MTC cases with distant metastasis. According to this study, the cancer-specific- survival for patients with MTC and isolated liver metastasis were reported to be 100.0% at one, three, five, and 10 years, respectively. However, the corresponding values for patients with multi-organ metastasis were 84.6%, 53.8%, 46.2%, and 30.8%, respectively [9] In our patient, diagnostic evaluation for metastatic MTC including whole body scan, abdominopelvic CT, 68 Ga-Dotatate PET-CT, and EUS could not detect any focus of malignancy.
Metastatic liver NET of unknown origin versus primary hepatic NET (PHNET) might be considered as the second scenario. The liver is the most common site for metastatic NET of the gastrointestinal tract and pancreas in the majority of cases [10, 11], but PHNET is a rare disease with an incidence rate of 0.17% [12]. PHNETs are slightly predominant in females with a mean age of 49.8 years [13]. Tumor markers including CEA, CA19-9, and alpha-fetoprotein are usually normal; even elevated values are not sufficient for diagnosis of PHNET; furthermore, imaging studies usually reveal nonspecific hypervascular hepatic mass [12]. Therefore, the diagnosis of PHNET is confirmed by a combination of radiological findings and IHC stainings, as well as the exclusion of an extrahepatic primary lesion [14]. Serum CgA, a hydrophilic glycoprotein, is a useful marker with a sensitivity of 85.5% and specificity of 98.5% in follow-up of all NETs [15]. The prognosis of surgically resectable cases of primary hepatic neuroendocrine carcinoma (PHNEC) is favorable [16]. Zhang et al. in 58 cases of PHNEC reported that the 5-year survival rate and mean survival were 80% and 148 months, respectively [17]. Regarding normal radiological findings and the normal value of serum CgA, PHNET or metastatic liver NET could not contribute to the significant increase in liver enzymes and subsequently chronic liver disease in our patient.
The last scenario is reactive neuroendocrine differentiation of cholangiocytes after cholestatic liver injury. The epithelial cell lining of intra- and extrahepatic bile ducts i.e., cholangiocytes, participate in bile formation via secretion of water, bicarbonate, and chloride ion. In cholangiopathies including primary biliary cholangitis or primary sclerosing cholangitis, these cells are pivotal, revealing various specific reactions including non-neoplastic proliferation, epithelial-to-mesenchymal transition, and achieving neuroendocrine phenotype [15]. These activated proliferating cholangiocytes may achieve new phenotypes and generate different soluble mediators to regulate its proliferation as well as survival, apoptosis, and function of parenchymal and mesenchymal liver cells and even elements of the immune system in an autocrine/paracrine manner. Hence, this component not only plays role in the development and progression of liver damage but also in repair mechanisms in the course of chronic liver disease [18]. Likewise, in hepatocyte damage, cholangiocytes cooperate in liver regeneration, secreting several substances including cytokines, growth factors, neuropeptides, and hormones. We emphasized that in the presence of neuroendocrine-like cells in liver biopsy in a patient with chronic liver diseases, after excluding NET using biochemical and radiological modalities, the ability of hepatic cholangiocytes to achieve neuroendocrine phenotype mimicking NET in liver biopsy may be considered [19].
In conclusion, we reported a case of MTC with a highly elevated serum Ctn level at presentation, being in remission both clinically and para-clinically after total thyroidectomy for more than 6 years. During the patient follow-up, due to progressive rising levels of liver enzymes with a predominantly cholestatic pattern, a liver biopsy was performed that revealed nests of neuroendocrine-like cells in the background of PBC. To justify this medical dilemma, we approached the patient as a multidisciplinary team consisting of a hepatologist, oncologist, radiologist, and pathologist. Considering normal tumor biomarkers and unremarkable imaging, metastatic MTC, as well as PHNET, was ruled out and our findings herald acquiring neuroendocrine phenotype by proliferating cholangiocytes. In this case, we highlighted the importance of a multidisciplinary team approach consist of healthcare professionals from different fields to determine the diagnosis and treatment plan in patients with an unusual manifestation of disease. As limitation, we did not perform the calcium-loaded calcitonin test in the management of this patient. However, the significant decrement of serum calcitonin level in immediate post-operative state as well as nearly undetectable levels of serum calcitonin during the 6-year follow-up, make MTC recurrence as an unlikely condition. However, due to a significant decrease in serum calcitonin level immediately after surgery and an undetectable level of it during the 6-year follow-up, recurrence of medullary thyroid cancer is very unlikely.
Availability of data and materials
Data are available from the corresponding author on reasonable request.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ATA:
-
American thyroid association
- CEA:
-
Carcinoembryonic antigen
- CgA:
-
Chromogranin A
- CT:
-
Computed tomography
- Ctn:
-
Calcitonin
- EUS:
-
Endoscopic ultrasound
- FNA:
-
Fine needle aspiration
- GGT:
-
Gamma-glutamyl transferase
- IHC:
-
Immunohistochemical
- MTC:
-
Medullary thyroid carcinoma
- NET:
-
Neuroendocrine tumor
- PBC:
-
Primary biliary cholangitis
- PET:
-
Positron emission tomography
- PHNET:
-
Primary hepatic neuroendocrine tumor
- RET:
-
Rearranged during transfection
- Tg:
-
Thyroglobulin
References
Ball DW. Medullary thyroid cancer: monitoring and therapy. Endocrinol Metab Clin North Am. 2007;36(3):823–37.
Tofail T, Fariduddin M, Haq T, Selim S, Jahan S, Khan MA, Mustari M, Banu H, Alam R, Joarder A. Metastatic medullary thyroid carcinoma with normal serum calcitonin levels. AACE Clin Case Rep. 2018;4(6):e439–42.
Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, Crocetti U, Attard M, Maranghi M, Torlontano M. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007;92(2):450–5.
Al-Salameh A, Baudry C, Gautier JF, Toubert M-E, Bihan H, Cohen R. Late liver metastasis of medullary thyroid cancer with low calcitonin levels—successfully cured by radiofrequency. Endokrynol Pol. 2016;67(3):326–9.
Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: the American Thyroid Association Guidelines Task Force on medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610.
Gambardella C, Offi C, Patrone R, Clarizia G, Mauriello C, Tartaglia E, Di Capua F, Di Martino S, Romano RM, Fiore L. Calcitonin negative Medullary Thyroid Carcinoma: a challenging diagnosis or a medical dilemma? BMC Endocr Disord. 2019;19(1):1–12.
Raue F, Frank-Raue K. Long-term follow-up in medullary thyroid carcinoma. Medullary Thyroid Carcinoma: Biology–Management–Treatment. 2015;204:207-25.
Censi S, Cavedon E, Watutantrige-Fernando S, Barollo S, Bertazza L, Manso J, Iacobone M, Nacamulli D, Galuppini F, Pennelli G. Unique Case of a Large Indolent Medullary Thyroid Carcinoma: Time to Reconsider the Medullary Thyroid Adenoma Entity? European Thyroid Journal. 2019;8(2):108–12.
Park H, Yang H, Heo J, Kim TH, Kim SW, Chung JH. Long-Term Outcomes and Causes of Death among Medullary Thyroid Carcinoma Patients with Distant Metastases. Cancers. 2021;13(18):4670.
Chen N, Slater K. Primary hepatic neuroendocrine tumours—Case series of a rare malignancy. Int J Surg Case Rep. 2019;55:145–8.
Chen Z, Xiao HE, Ramchandra P, Huang HJ. Imaging and pathological features of primary hepatic neuroendocrine carcinoma: An analysis of nine cases and review of the literature. Oncol Lett. 2014;7(4):956–62.
Wang L-X, Liu K, Lin G-W, Jiang T. Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging. 2015;15(1):1–7.
Lin C-W, Lai C-H, Hsu C-C, Hsu C-T, Hsieh P-M, Hung K-C, Chen Y-S. Primary hepatic carcinoid tumor: a case report and review of the literature. Cases J. 2009;2(1):1–6.
Jia C, Zhang Y, Xu J, Sun K. Experience in primary hepatic neuroendocrine tumor. Turk J Gastroenterol. 2012;23(5):546–51.
Lyubimova N, Churikova T, Kushlinskii N. Chromogranin as a biochemical marker of neuroendocrine tumors. Bull Exp Biol Med. 2016;160(5):702–4.
Park CH, Chung JW, Jang SJ, Chung MJ, Bang S, Park SW, Song SY, Chung JB, Park JY. Clinical features and outcomes of primary hepatic neuroendocrine carcinomas. J Gastroenterol Hepatol. 2012;27(8):1306–11.
Zhang A, Xiang J, Zhang M, Zheng S. Primary hepatic carcinoid tumours: clinical features with an emphasis on carcinoid syndrome and recurrence. J Int Med Res. 2008;36(4):848–59.
Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132(1):415–31.
Scrushy M, O'Brien A, Glaser S. Recent advances in understanding bile duct remodeling and fibrosis. F1000Res. 2018;7(1165).
Acknowledgements
We would like to thank the patient for agreeing to write her case as a report. We gratefully acknowledge members of the pathology department of MEHRAD and PARS Hospitals, we would like to thank Dr. azizollah Abbasi Dezfooli, the professor of thoracic surgery, shahid Beheshti university of medical science for surgical management of the patient.
Funding
None.
Author information
Authors and Affiliations
Contributions
F.R, F.H, and M.T wrote the case report. F. R, F.H performed the data collection. F. R, M.T, F.A reviewed the paper for intellectual content. All authors critically revised the manuscript, read and approved its final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient for representing this case report. A copy of the written consent is available for review.
Consent for publication
Written informed consent for publication was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’ s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rahmani, F., Tohidi, M., Azmoudeh-Ardalan, F. et al. Diagnostic dilemma in a patient with history of medullary thyroid carcinoma and abnormal serum liver enzymes; a case report with six years follow up. BMC Endocr Disord 23, 186 (2023). https://doi.org/10.1186/s12902-023-01439-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01439-7