Abstract
Chronic inflammation is an important pathway for obesity to harm health, the aggravation of chronic inflammation occurs without clinical symptoms. BMI is closely related to chronic inflammation, and it is a predictive factor of chronic inflammation, but the following questions remain unanswered: Are the effects of chronic inflammation on different BMI intervals consistent? Are the effects of BMI on chronic inflammation consistent between male and female? This study aimed to explore the threshold effect, and sex characteristics of the relationship between chronic inflammation and BMI. Methods: People with normal weight, overweight, and obesity were selected as subjects for cross-sectional study. BMI, hs-CRP, adiponectin and irisin was tested. Multiple regression analysis and generalized additive models were used to examine the association between hs-CRP and BMI. Results: 119 adults were recruited (normal weight: n = 30, 28.1 ± 7.65 years, BMI: 22.04 ± 1.55; overweight: n = 29, 27.45 ± 7.47 years, BMI: 26.11 ± 1.22; and obesity: n = 60, 28.82 ± 6.05 years, BMI: 33.68 ± 3.57). After adjusting for age and sex, BMI was found to be positively associated with the chronic inflammatory marker hs-CRP (β = 0.45; P < 0.001), which had a threshold effect on hs-CRP. Positive correlation with hs-CRP was observed for BMI of > 24.6 (β = 0.54; P < 0.001) but not for BMI of 18.5–25.4 (β = −0.02; P > 0.05). The pro-inflammatory effect caused by BMI increase in female (β = 0.56; P < 0.001) was higher than that in male (β = 0.38; P < 0.001). When BMI was greater than 33, a positive correlation with hs-CRP was observed in female (β = 0.97; P < 0.001) but not in male (β = 0.14; P > 0.05).
Conclusions: BMI has a threshold effect on chronic inflammation, BMI greater than 24.3 is positively correlated with hs-CRP. BMI in 18.5–24.3 is not correlated with hs-CRP. Furthermore, when the BMI greater than 33, hs-CRP is not positively correlated with BMI in male, whereas the pro-inflammatory effect of BMI increase becomes greater in female.
Highlights:
• BMI has a threshold effect on chronic inflammation. BMI in 18.5–24.3 is not correlated with chronic inflammation, and BMI greater than 24.3 is positively correlated with chronic inflammation.
• The pro-inflammatory effect caused by BMI increase in female is higher than that in male. In particular, when the BMI is greater than 33, chronic inflammation is not positively correlated with BMI in male, whereas the pro-inflammatory effect of BMI increase becomes greater in female.
Similar content being viewed by others
Introduction
The incidence of obesity, obesity-related metabolic diseases, tumors, and other diseases has increased sharply worldwide [1,2,3,4]. These diseases bring huge burden to families and society [5,6,7]. Obesity endangers health through chronic inflammation [8]. Garvin et al. [9] showed that the increase in chronic inflammation markers IL-6 and CRP is related to obesity. Ying W et al. [10] found that chronic inflammation associated with obesity. The development of chronic inflammation in people with obesity often lacks obvious clinical symptoms. Therefore, understanding the relationship between BMI increase and aggravated chronic inflammation plays an important role in preventing the occurrence of obesity and obesity-related diseases.
In general, chronic inflammation is evaluated on the basis of the intermediate products or outcome products of the inflammatory reaction process[11]. TNF-α is primarily produced by adipocytes and macrophages in adipocytes, which can reveal chronic inflammation by reflecting the activation of these cells [12, 13]. hs-CRP is produced in the liver stimulated by many inflammatory mediators [11], which reflects a wide and diffuse range of inflammation. In the study of obesity and chronic inflammation, some scholars used TNF-α [14], CRP [15], and hs-CRP to evaluate chronic inflammation [16, 17]. TNF-α and hs-CRP are reliable markers for evaluating obesity-related chronic inflammation [18, 19].
BMI increase is accompanied by the aggravation of chronic inflammation [20, 21]. Scholars included people with normal weight, overweight, and obesity as subjects and found that hs-CRP increased with BMI [22]. BMI is considered to be a risk factor for predicting chronic inflammation. Many studies have shown that chronic inflammation can show different characteristics in different sex. Chang et al. found significant differences in hs-CRP among different sex[23], which may be related to sex differences in chromosomes, sex hormones, and other factors[24]. Therefore, sex factors should be paid attention to when exploring chronic inflammation. Obesity-related chronic inflammation is sex specific[25]. In addition, chronic inflammation is associated with obesity, and this association might be modified by sex[26]. Although the relationship between chronic inflammation and BMI has been widely studied, the following questions remain unanswered: Are the effects of chronic inflammation on different BMI intervals consistent? Are the effects of BMI on chronic inflammation consistent between male and female? Solving these problems may play an important role in the targeted prevention and treatment of chronic inflammation aggravation caused by BMI increase.
People with normal weight, overweight, and obesity were selected as subjects for this cross-sectional study. The influence of BMI on chronic inflammation was analyzed on the basis of testing age, height, weight, and chronic inflammation markers. The threshold effect of BMI on chronic inflammation was determined by adjusting age and sex through regression analysis.
Materials and methods
This study is a prospective cross-sectional study, and follow the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Supplementary Table 1).
Participants and recruitment
The investigation proceeded by means of a successive sampling study. Volunteers from the Yangzhou Lipan weight loss training camp were recruited from May 2020 to August 2020. The inclusion criteria were as follows: age ≥ 18 years old, BMI ≥ 18.5, regardless of sex. The exclusion criteria were as follows: those with inflammatory events, infectious diseases, long-term medication, hypertension, diabetes, professional physical training, and sports contraindications. During training, the health records of the volunteers were obtained. This study was ethically approved by Fujian Normal University Ethics Committee with clinical trial registration number ChiCTR2200058959. All participants signed an informed consent form for this study.
Outcome measures
Key outcomes included basic information, irisin, adiponectin, and hs-CRP. Basic information included name, sex, age, height, weight, physical examination, disease, medication, and sports injury. The methods for testing key outcome indicators are based on literature [27, 28].
Body composition assessment
Height (0.1 cm) was measured using an instrument, and weight was assessed using a body composition analyzer. During the test, the participants wore slippers, hats, and single clothes. Test conditions were maintained before and after the repeated test. Body weight was presented in kilograms (kg). BMI was calculated as weight (kg) ÷ [height (m)]2. The Chinese BMI criteria[29, 30] were as follows: normal weight: 18.5 ≤ BMI < 24, overweight: 24 ≤ BMI < 28, obesity: BMI ≥ 28.
Testing of blood chronic inflammatory factors such as hs-CRP, adiponectin, and irisin
Blood indexes were measured as follows. Blood was extracted from the volunteers on an empty stomach from 7:00 to 8:00 in the morning of the second day of training. They fasted after 22:00 in the previous day. Five milliliters of blood was collected from the elbow vein of negative pressure blood collection vessel containing a separating gel coagulant. After the sample was allowed to stand at room temperature for 30 min, it was centrifuged at 3000 rpm for 5 min. The first 1 mL serum was removed and placed into a 1.5 mL antifreeze centrifuge tube. Liquid nitrogen preservation was performed to test irisin and adiponectin. In addition, relevant ELISA kits were purchased from Wuhan Yilairuite Biotechnology Co., Ltd. The second serum was kept in the blood collection vessel at 4 ℃, and hs-CRP was tested in the Nanjing Aidikang medical laboratory center.
Statistical analysis
All continuous variables were expressed as means ± standard deviations, and categorical data were presented as frequencies and percentages, the results of regression analysis are described as regression coefficient β (95% confidence interval) P value. All tests were evaluated for normality using Shapiro-Wilk statistics. All data were included in the final analyses.
The Chi-squared test was used to compare the differences in categorical variables. One-way ANOVA was used to compare the differences between different groups of continuous variables and normal distribution, and the Kruskal–Wallis rank sum test was used for those with non-normal distribution. Smooth curve fitting was performed according to the generalized additive model. The linear relationship was described by a univariate linear regression model. The threshold effect analysis was used to describe the linear/nonlinear relationship between the concentration of hs-CRP and BMI. Threshold effect analysis identifies nonlinear relationship key point (K) exploring the relationship hs-CRP and BMI, threshold effect model was a two-piece-wise regression model (< K effect 1 and > K effect 2) [31]. The interfering variables are adjusted to reduce bias when conducting regression models. Using sex as subgroups, analyze sex characteristics of BMI affecting hs-CRP through Smooth curve fitting and threshold effect analysis. A multiple regression analysis model with covariates was powered using the predictor variables established in each of the multivariable model and performed using G-power3.1.9.2 [32, 33]. R2 was used to measure the effect quantity [34]. According to Cohen (1998) [35], when R2 is 0.13 in regression analysis, it can achieve medium test efficiency. The effect size f2 of the regression model was calculated as 0.15 with R2 = 0.13. α Error was set to 0.05; power (1-β Error) was set to 0.9, and the estimated target sample size was n = 108 participants. Use regression imputation to fill in missing data. The data were processed and analyzed by statistical packages R (R foundation, version 3.1.2) and empower (R). P < 0.05 and P < 0.01 indicate that the difference is statistically significant.
Results
Study population characteristics
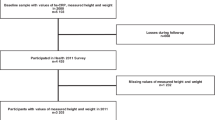
119 adults (28.30 ± 6.80 years; male, n = 58; female, n = 61) were included in the final analysis (Fig. 1). Differences in participant characteristics across the three study cohorts are presented in Tables 1 and 2. No significant difference in age, height, irisin, and adiponectin was observed among the three groups (P > 0.05). Significant differences in body weight, BMI, and chronic inflammation marker hs-CRP were found among the three groups (P < 0.01). Significant differences in body weight and height were found between male and female (P < 0.01). Furthermore, no significant difference in age, BMI, irisin, adiponectin, and hs-CRP was observed between male and female (P > 0.05).
Relationship between BMI and chronic inflammation
Using hs-CRP as the dependent variable and BMI as the independent variable, regression analysis showed that BMI was positively correlated with hs-CRP (β = 0.44; P < 0.01). After adjustment for sex or age or sex and age, BMI was found to be positively correlated with hs-CRP (β = 0.44, P < 0.01; β = 0.44, P < 0.01; β = 0.45, P < 0.01; Table 3).
The generalized additive models were then used for threshold analysis, and the results showed the threshold effect of BMI on hs-CRP with a BMI influence point at 24.3. Therefore, hs-CRP was positively correlated with BMI of > 24.3 (β = 0.51, P < 0.001) but not with BMI of 18.5–24.3 (β = −0.01; P > 0.05). Moreover, after adjustment for sex or age or sex and age, BMI had a threshold effect on hs-CRP with BMI influence point at 24.3, 24.6, and 24.6 (Table 3).
Curve fitting of BMI on chronic inflammation
Using hs-CRP as the dependent variable, BMI as the independent variable, and age and sex as the adjustment variables, curve fitting showed that BMI had a curve relationship with hs-CRP (Fig. 2). By contrast, using hs-CRP as the dependent variable, BMI as the independent variable, and age as the adjustment variable, curve fitting showed that BMI had a different curve relationship with hs-CRP in male and female when BMI was more than 33 (Fig. 3).
Using hs-CRP as the dependent variable and BMI as the independent variable, regression analysis showed that BMI was positively correlated with hs-CRP (β = 0.36, P < 0.01 in male and β = 0.56, P < 0.01 in female). After adjustment for age, BMI was found to be positively correlated with hs-CRP (β = 0.38, P < 0.01 in male and β = 0.56, P < 0.01 in female; Table 4). The generalized additive models were then used for threshold analysis, and the K value was set to 33 (Fig. 3). The results showed that hs-CRP was not correlated with BMI > 33 (β = 0.08, P > 0.05 in male), but hs-CRP was positively correlated with BMI > 33 (β = 0.97, P < 0.01 in female). After adjustment for age, hs-CRP was not correlated with BMI > 33 (β = 0.14, P > 0.05 in male), but hs-CRP was positively correlated with BMI > 33 (β = 0.97, P < 0.01 in female; Table 4).
Discussion
This study found that BMI is an independent risk factor for chronic inflammation. However, great differences in body health level occur in different BMI intervals [36, 37]. Wedell-Neergaard AS[38] found that BMI is not related to chronic inflammation, possibly because the BMI of the included population is concentrated between 22 and 26. Samara A[39] found that BMI is significantly correlated with chronic inflammation, and the BMI of the included population is between 19 and 33. It can be seen that the relationship between BMI and chronic inflammation varies among different BMI intervals. This study found a segmentation effect between the change of chronic inflammation marker hs-CRP and BMI. Chronic inflammation was not related to BMI ranging from 18.5 to 24.3, but it significantly increased in individuals with BMI of > 24.3. The accumulation of chronic inflammation occurs in the overweight stage. Therefore, attention must be paid to the overweight stage to prevent the aggravation of chronic inflammation. In previous reports, the blood CRP content in the overweight group was higher than that in the standard study group[40], and the abnormal rate of CRP content in the blood of 33 overweight volunteers was higher than that in the normal weight group [41]. These results showed that chronic inflammation has begun to gradually appear in the overweight stage, which is consistent with the present results. The overweight stage is the beginning of the accumulation and intensification of chronic inflammation and the beginning of excess energy. Excess energy may initially induce chronic inflammation, prompt the hypertrophy of adipocytes, lead to the relative hypoxia of intracellular organelles, increase the oxidative stress of the endoplasmic reticulum, and cause the release of proinflammatory factors into the blood [42]. Excess nutrition can cause lipid metabolism disorder and excessive fatty acid accumulation, which can promote the secretion of inflammatory factors by immune cells and cause slow inflammation. Combined with the abovementioned findings, the present results showed that chronic inflammation is aggravated when BMI is > 24.3. Therefore, controlling body weight in the overweight stage is crucial to prevent the aggravation of chronic inflammation.
Significant differences in the immune system are observed between adult male and female[43], which makes the characteristics of chronic inflammation different between the two sex[24, 26, 44]. This study found that the pro-inflammatory effect of BMI increase was higher in female than in male. Moreover, when BMI > 33, the pro-inflammatory effect of BMI increase is greater in female, and no aggravating trend of chronic inflammation was observed in male. Therefore, the mechanism of chronic inflammation caused by BMI increase in different sex may be different. BMI increase is a process of energy accumulation, and the accumulated excess energy can be stored in adipose tissue. Adipose tissue is mostly composed of adipocytes, and adipose tissue mass is determined on the basis of the number and size of adipocytes. Female tends to show an increase in fat mass based on an increase in the number and size of adipocytes, whereas men mostly show an increase in the size of adipocytes, predisposing female to systemic inflammation[45]. Therefore, the different anti-inflammatory characteristics of adipose tissue in different sex may cause the different anti-inflammatory efficacy of BMI increase in different sex.
Some studies found that the expression of adiponectin and irisin may be different between male and female, and they both have anti-inflammatory effects[46,47,48,49,50]. This study showed no significant difference in the concentration of irisin and adiponectin between sex, and the average concentration of female was lower than that of male. Therefore, the stronger pro-inflammatory effect of BMI increase in female may be related to the decreased protection of anti-inflammatory effect in adiponectin and irisin. Ter Horst et al.[51] have shown that females usually show lower adiponectin concentrations, whereas the decrease of serum adiponectin concentration is related to chronic inflammation [52]. In the healthy population, the concentration of serum irisin in female is lower than that in male[53]. Irisin alleviates inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling [47, 54,55,56]. Insulin also plays an important role in regulating chronic inflammation. Wiebe N et al. [57] showed that when BMI is greater than 35, female’s fasting insulin levels are lower than male’s, and insulin has a certain anti-inflammatory effect, which may be the mechanism by which female exhibit greater pro-inflammatory effects at larger BMI.
Although adipose tissue, adiponectin, irisin, and insulin partially explain that the pro-inflammatory effect of BMI increase in female is higher than that in male, there is no direct proof that these indicators can initiate the inflammatory cascade in this study. systematic and large-scale investigations are necessary to determine the factors influencing the response of female with obesity to inflammation.
This study also has some limitations. The sample size is small, which only meets the medium efficiency of statistics. People with BMI less than 18.5 were not included, and the change law of chronic inflammation was not reflected in this range. Moreover, there are still some uncontrollable interfering factors in exploring the relationship between chronic inflammatory characteristics and BMI. Although our multiple regression analysis was adjusted, some residual confounding factors cannot be ruled out. In particular, not all potential confounders were considered in the adjustment, such as psychosocial factors and nutritional status, which could affect chronic inflammation.
Conclusions
Within a certain range, BMI is an important risk factor for chronic inflammation, BMI has a threshold effect on chronic inflammation, BMI greater than 24.3 is positively correlated with hs-CRP. BMI in 18.5–24.3 is not correlated with hs-CRP. Moreover, when BMI is greater than 33, there may be sex differences in the relationship between BMI and chronic inflammation.
Data availability
The datasets used in the analyses described in this study are available from the corresponding author on reasonable request.
References
Kim MS, Kim WJ, Khera AV, Kim JY, Yon DK, Lee SW, Shin JI, Won HH. Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and mendelian randomization studies. Eur Heart J. 2021;42(34):3388–403.
Katta N, Loethen T, Lavie CJ, Alpert MA. Obesity and Coronary Heart Disease: Epidemiology, Pathology, and coronary artery imaging. Curr Probl Cardiol. 2021;46(3):100655.
Elagizi A, Kachur S, Carbone S, Lavie CJ, Blair SN. A review of obesity, physical activity, and Cardiovascular Disease. Curr Obes Rep. 2020;9(4):571–81.
Dwivedi AK, Dubey P, Cistola DP, Reddy SY. Association between obesity and Cardiovascular Outcomes: updated evidence from Meta-analysis studies. Curr Cardiol Rep. 2020;22(4):25.
Herrera-Espiñeira C, de Pascual YMAM, López-Morales M, Díaz Jiménez P, Rodríguez Ruiz A, Expósito-Ruiz M. Differences in Dietary Habits, Physical Exercise, and quality of life between patients with obesity and overweight. Healthc (Basel) 2021, 9(7).
Stephenson J, Smith CM, Kearns B, Haywood A, Bissell P. The association between obesity and quality of life: a retrospective analysis of a large-scale population-based cohort study. BMC Public Health. 2021;21(1):1990.
van der Heijden LB, Feskens EJM, Raat H, Janse AJ. Quality of life of children and adolescents with clinical obesity, perspectives of children and parents. Obes Res Clin Pract. 2021;15(5):466–72.
Simoes E, Correia-Lima J, Sardas L, Storti F, Otani T, Vasques DAC, Otani VHO, Bertolazzi P, Kochi C, Seelaender M, et al. Sex dimorphism in inflammatory response to obesity in childhood. Int J Obes. 2021;45(4):879–87.
Garvin P, Nilsson E, Ernerudh J, Kristenson M. The joint subclinical elevation of CRP and IL-6 is associated with lower health-related quality of life in comparison with no elevation or elevation of only one of the biomarkers. Qual Life Res. 2016;25(1):213–21.
Ying W, Lee YS, Dong Y, Seidman JS, Yang M, Isaac R, Seo JB, Yang BH, Wollam J, Riopel M, et al. Expansion of Islet-Resident Macrophages leads to inflammation affecting beta cell proliferation and function in obesity. Cell Metab. 2019;29(2):457–74. e455.
Setty BNY, Betal SG, Miller RE, Brown DS, Meier M, Cahill M, Lerner NB, Apollonsky N, Stuart MJ. Relationship of Omega-3 fatty acids DHA and EPA with the inflammatory biomarker hs-CRP in children with sickle cell anemia. Prostaglandins Leukot Essent Fat Acids. 2019;146:11–8.
Aladhami AK, Unger CA, Ennis SL, Altomare D, Ji H, Hope MC 3rd, Velázquez KT, Enos RT. Macrophage tumor necrosis factor-alpha deletion does not protect against obesity-associated metabolic dysfunction. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2021;35(7):e21665.
Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–26.
Wood LG, Li Q, Scott HA, Rutting S, Berthon BS, Gibson PG, Hansbro PM, Williams E, Horvat J, Simpson JL, et al. Saturated fatty acids, obesity, and the nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome in asthmatic patients. J Allergy Clin Immunol. 2019;143(1):305–15.
Unamuno X, Gómez-Ambrosi J, Ramírez B, Rodríguez A, Becerril S, Valentí V, Moncada R, Silva C, Salvador J, Frühbeck G et al. Dermatopontin, a Novel Adipokine promoting adipose tissue extracellular matrix remodelling and inflammation in obesity. J Clin Med 2020, 9(4).
Porter Starr KN, Orenduff M, McDonald SR, Mulder H, Sloane R, Pieper CF, Bales CW. Influence of Weight Reduction and enhanced protein intake on biomarkers of inflammation in older adults with obesity. J Nutr Gerontol Geriatr. 2019;38(1):33–49.
Yan P, Xu Y, Zhang Z, Gao C, Zhu J, Li H, Wan Q. Decreased plasma neuregulin 4 levels are associated with peripheral neuropathy in chinese patients with newly diagnosed type 2 diabetes: a cross-sectional study. Cytokine. 2019;113:356–64.
Letchumanan I, Arshad MKM, Gopinath SCB. Nanodiagnostic attainments and clinical perspectives on C-Reactive protein: Cardiovascular Disease Risks Assessment. Curr Med Chem. 2021;28(5):986–1002.
Kenđel Jovanović G, Mrakovcic-Sutic I, Pavičić Žeželj S, Šuša B, Rahelić D, Klobučar Majanović S. The efficacy of an energy-restricted anti-inflammatory Diet for the management of obesity in younger adults. Nutrients 2020, 12(11).
Karczewski J, Śledzińska E, Baturo A, Jończyk I, Maleszko A, Samborski P, Begier-Krasińska B, Dobrowolska A. Obesity and inflammation. Eur Cytokine Netw. 2018;29(3):83–94.
Fuentes GC, Castañer O, Warnberg J, Subirana I, Buil-Cosiales P, Salas-Salvadó J, Corella D, Serra-Majem L, Romaguera D, Estruch R, et al. Prospective association of physical activity and inflammatory biomarkers in older adults from the PREDIMED-Plus study with overweight or obesity and metabolic syndrome. Clin Nutr. 2020;39(10):3092–8.
McDade TW, Meyer JM, Koning SM, Harris KM. Body mass and the epidemic of chronic inflammation in early mid-adulthood. Soc Sci Med. 2021;281:114059.
Chang E, Varghese M, Singer K. Gender and sex differences in adipose tissue. Curr Diab Rep. 2018;18(9):69.
Zore T, Palafox M, Reue K. Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes? Mol metabolism. 2018;15:35–44.
Kohn JN, Cabrera Y, Dimitrov S, Guay-Ross N, Pruitt C, Shaikh FD, Hong S. Sex-specific roles of cellular inflammation and cardiometabolism in obesity-associated depressive symptomatology. Int J Obes. 2019;43(10):2045–56.
Thorand B, Baumert J, Döring A, Herder C, Kolb H, Rathmann W, Giani G, Koenig W. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184(1):216–24.
Kakino A, Fujita Y, Ke LY, Chan HC, Tsai MH, Dai CY, Chen CH, Sawamura T. Adiponectin forms a complex with atherogenic LDL and inhibits its downstream effects. J Lipid Res. 2021;62:100001.
Garcés MF, Peralta JJ, Ruiz-Linares CE, Lozano AR, Poveda NE, Torres-Sierra AL, Eslava-Schmalbach JH, Alzate JP, Sánchez AY, Sanchez E, et al. Irisin levels during pregnancy and changes associated with the development of preeclampsia. J Clin Endocrinol Metab. 2014;99(6):2113–9.
Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in chinese adults–study on optimal cut-off points of body mass index and waist circumference in chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–92.
Lin L, Chen CZ, Yu XD. [The analysis of threshold effect using Empower Stats software]. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34(11):1139–41.
Muller K. Statistical power analysis for the behavioral Sciences. Technometrics. 1989;31(4):499–500.
Gauss T, Ageron FX, Devaud ML, Debaty G, Travers S, Garrigue D, Raux M, Harrois A, Bouzat P. Association of Prehospital Time to In-Hospital trauma mortality in a physician-staffed Emergency Medicine System. JAMA Surg. 2019;154(12):1117–24.
Correll J, Mellinger C, McClelland GH, Judd CM. Avoid Cohen’s ‘Small’, ‘Medium’, and ‘Large’ for Power Analysis. Trends Cogn Sci. 2020;24(3):200–7.
Cohen J. Statistical power analysis for the behavioral Sciences. New Jersey: L. Erlbaum Associates; 1988.
Fiori F, Bravo G, Parpinel M, Messina G, Malavolta R, Lazzer S. Relationship between body mass index and physical fitness in italian prepubertal schoolchildren. PLoS ONE. 2020;15(5):e0233362.
Rafatifard M, Mazloomy Mahmoodabad SS, Fallahzadeh H. The physical activity level and aerobic capacity estimation (VO2max) among the administrative staff of the Pars Special Economic Energy Zone (Assaluyeh, Iran) with different BMIs. Horm Mol Biol Clin Investig 2019, 38(3).
Wedell-Neergaard AS, Eriksen L, Grønbæk M, Pedersen BK, Krogh-Madsen R, Tolstrup J. Low fitness is associated with abdominal adiposity and low-grade inflammation independent of BMI. PLoS ONE. 2018;13(1):e0190645.
Samara A, Pfister M, Marie B, Visvikis-Siest S. Visfatin, low-grade inflammation and body mass index (BMI). Clin Endocrinol (Oxf). 2008;69(4):568–74.
Kim MJ, Hwang JH, Ko HJ, Na HB, Kim JH. Lemon detox diet reduced body fat, insulin resistance, and serum hs-CRP level without hematological changes in overweight korean women. Nutr Res. 2015;35(5):409–20.
Feidantsis K, Methenitis S, Ketselidi K, Vagianou K, Skepastianos P, Hatzitolios A, Mourouglakis A, Kaprara A, Hassapidou M, Nomikos T et al. Comparison of short-term hypocaloric high-protein diets with a hypocaloric Mediterranean diet: Effect on body composition and health-related blood markers in overweight and sedentary young participants. Nutr (Burbank Los Angeles Cty Calif) 2021, 91–2:111365.
Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–17.
Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294(2):102–10.
Lasselin J, Lekander M, Axelsson J, Karshikoff B. Sex differences in how inflammation affects behavior: what we can learn from experimental inflammatory models in humans. Front Neuroendocrinol. 2018;50:91–106.
Heo JW, Kim SE, Sung MK. Sex differences in the incidence of obesity-related gastrointestinal Cancer. Int J Mol Sci 2021, 22(3).
Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J, Mantzoros CS. Irisin in metabolic diseases. Endocrine. 2018;59(2):260–74.
Korta P, Pocheć E, Mazur-Biały A. Irisin as a multifunctional protein: implications for health and certain Diseases. Med (Kaunas) 2019, 55(8).
Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci 2017, 18(6).
Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8(3):1031–63.
da Silva Rosa SC, Liu M, Sweeney G. Adiponectin synthesis, secretion and extravasation from circulation to interstitial space. Physiol (Bethesda Md). 2021;36(3):134–49.
Ter Horst R, van den Munckhof ICL, Schraa K, Aguirre-Gamboa R, Jaeger M, Smeekens SP, Brand T, Lemmers H, Dijkstra H, Galesloot TE, et al. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol. 2020;40(7):1787–800.
Choi HM, Doss HM, Kim KS. Multifaceted physiological roles of adiponectin in inflammation and Diseases. Int J Mol Sci 2020, 21(4).
Ruan Q, Huang Y, Yang L, Ruan J, Gu W, Zhang X, Zhang Y, Zhang W, Yu Z. The effects of both age and sex on irisin levels in paired plasma and cerebrospinal fluid in healthy humans. Peptides. 2019;113:41–51.
Gonzalez-Gil AM, Elizondo-Montemayor L. The role of Exercise in the interplay between Myokines, Hepatokines, Osteokines, Adipokines, and modulation of inflammation for energy substrate redistribution and Fat Mass loss: a review. Nutrients 2020, 12(6).
Li Q, Tan Y, Chen S, Xiao X, Zhang M, Wu Q, Dong M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. J Recept Signal Transduct Res. 2021;41(3):294–303.
Restuccia R, Perani F, Ficarra G, Trimarchi F, Bitto A, di Mauro D. Irisin and vascular inflammation: Beneficial Effects of a healthy lifestyle beyond physical activity. Curr Pharm Design. 2021;27(18):2151–5.
Wiebe N, Muntner P, Tonelli M. Associations of body mass index, fasting insulin, and inflammation with mortality: a prospective cohort study. Int J Obes. 2022;46(12):2107–13.
Acknowledgements
The authors would like to thank all the participants for their valuable contribution.
Funding
The funded was provided by National Natural Science Foundation of China (31971099) and Jiangsu Federation of philosophy and Social Sciences (21WRA001). The funding bodies played no role in the design of the study and collection, analysis, interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, SL, CH, LF; methodology, SL, QM; investigation, SL, CH; data curation, SL, CH; writing—original draft preparation, SL, CH; supervision, CH. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The trial was ethically approved by Fujian Normal University Ethics Committee with clinical trial registration number ChiCTR2200058959. All participants signed an informed consent form for this study. All methods were performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki).
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liqiang, S., Fang-Hui, L., Minghui, Q. et al. Threshold effect and sex characteristics of the relationship between chronic inflammation and BMI. BMC Endocr Disord 23, 175 (2023). https://doi.org/10.1186/s12902-023-01396-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01396-1