Abstract
Background
Primary bilateral macronodular adrenocortical hyperplasia (PBMAH) is a highly heterogeneous disease with divergent manifestations ranging from asymptomatic subclinical Cushing syndrome (CS) to overt Cushing syndrome with severe complications. ARMC5 mutations occur in 20 to 55% PBMAH patients usually with more severe phenotypes. Different ARMC5 mutations might be associated with diverse phenotypes of PBMAH.
Case presentation
A 39-year-old man was admitted to our hospital with progressive weight gain and severe hypertension. He presented typical CS and its classical metabolic and bone complications like hypertension and osteoporosis. The laboratory results showed high levels of cortisol and low levels of ACTH. Low- and high-dosed dexamethasone suppression tests were negative. Contrast-enhanced computed tomography (CT) revealed multiple bilateral irregular macronodular adrenal masses. Adrenal venous sampling (AVS) confirmed that the right adrenal gland with larger nodules secreted more hormone that the left side did. Right adrenalectomy and subsequent contralateral subtotal resection were conducted. His blood pressure and CS symptoms as well as comorbidities including backache and muscle weakness improved. Whole exome sequencing identified one ARMC5 germline mutation (c.1855C > T, p. R619*), five ARMC5 somatic mutations (four novel mutations) in his right and left adrenal nodules.
Conclusions
This PBMAH patient was identified with one ARMC5 germline mutation and five different somatic ARMC5 mutations (four novel mutations) in the different nodules of the bilateral adrenal masses. AVS combined with CT imagine could be helpful to determine the dominant side for adrenalectomy. Genetic testing is important for the diagnosis and management of the patient with PBMAH.
Similar content being viewed by others
Background
Primary bilateral macronodular adrenocortical hyperplasia (PBMAH) is a highly heterogeneous disease and variable levels of cortisol produced by bilateral benign adrenocortical macronodules larger than 10 mm [1]. Patients with PBMAH present with divergent phenotypes ranging from asymptomatic subclinical Cushing syndrome (SCS) to overt Cushing syndrome (CS) with severe complications. Overt CS includes weight gain, proximal myopathy, buffalo neck, red stretch marks, skin fragility, easy bruising, hyperandrogenism, and classical metabolic and bone complications such as hypertension, diabetes, venous thrombo-embolism, osteopenia, and osteoporosis [2]. Most patients with clinical symptoms are diagnosed between 40 and 60 years of age and the diagnosis is often made only after several years or decades of disease progression indicating that the tumor growth and cortisol dysregulation progress slowly and silently [1]. Mild autonomous cortisol secretion is associated with absent or moderate symptoms, thus most PBMAH patients are identified incidentally during computed tomography and magnetic resonance imaging for unrelated conditions [2], which makes early diagnosis challenging.
The bilateral nature of the adrenal lesions and the description of familial cases of patients with PBMAH provide support for the hypothesis of a germline genetic predisposition [2]. Previous studies have shown that inactivating germline mutations of tumor suppressor genes including MEN1, APC and FH could result in PBMAH accompanied by other syndromic presentations such as primary hyperparathyroidism, neuroendocrine tumors, pituitary adenomas (MEN1 syndrome, multiple endocrine neoplasia type 1), colon polyps (FAP, familial adenomatous polyposis), leiomyomas, leiomyosarcomas and renal cancer (HLRCC, hereditary leiomyomatosis and renal cell cancer) [3]. ARMC5 pathogenic mutations occur in 20 to 55% of patients with PBMAH [1, 4, 5]. Noteworthy, patients carrying ARMC5 mutations usually have earlier onset, bigger nodules, higher cortisol levels, and more severe symptoms [4]. It has been noticed that some symptoms appear to be ARMC5 mutation-specific [6]. PBMAH patients with ARMC5 mutations are more often treated surgically [2]. From this point of view, identifying PBMAH patients with ARMC5 mutations might be important for the treatment decision-making. Here we report a PBMAH patient carrying one germline mutation and five somatic mutations in ARMC5.
Case presentation
Clinical features
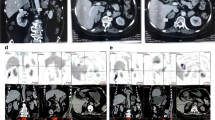
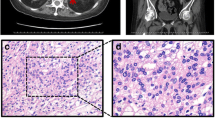
A 39-year-old male was admitted to our hospital with progressive weight gain for 8 years from 60 kg (BMI: 24.7 kg/m2) to 71.8 kg (BMI: 29.4 kg/m2) and severe hypertension refractory to antihypertensive drugs for 2 years (150–180/100–120 mmHg). Physical examination showed multiple clinical manifestations of CS such as moon face, centripetal obesity, abdominal purple striae, limb edema, and muscle weakness. The hormonal work-up on admission confirmed significantly increased serum cortisol and urinary free cortisol (UFC) as shown in Table 1. In addition, his plasma ACTH levels were suppressed markedly at all time points, especially at 16:00 (2.69 pg/mL, normal ranges: 10.7–30.5 pg/mL). The results of LD-DST and HD-DST were both negative. All these findings suggested that his hypercortisolism was ACTH-independent. In addition, he had low level of testosterone (0.1 ng/ml, normal range: 1.75–7.81 ng/mL), which could happen due to decreased gonadal function in severe Cushing syndrome, while the levels of LH (1.62mIU/mL, 1.24–8.62mIU/mL) and FSH (4.21mIU/mL, normal range: 1-8mIU/mL) are normal. Contrast-enhanced CT showed bilateral adrenal multiple irregular macronodular masses (right adrenal: 7.2 × 5.2 × 5.1 cm, left adrenal: 6.9 × 4.5 × 3.2 cm) (Fig. 1). Positron emission tomography/computed tomography (PET/CT) revealed vertebral (T4-T6) compression, osteoporosis, enlarged heart, as well as multiple nodules in the bilateral adrenal masses. The lower bone mineral density (BMD) further substantiated the osteoporosis. Adrenal venous sampling (AVS) was conducted to evaluate the predominant side of cortisol production (Table 2). Selectivity indexes (SI) were 1.77 and 3.04 in the left and right adrenals, respectively, indicating the success of AVS cannulation (SI cutoff of 1.4) [7]. However, there was no lateralization according to the lateralization index (LI < 4). Based on the absolute serum cortisol level, the right adrenal with larger nodules was confirmed to produce more cortisol than the left. Considering the AVS result and the sizes of the adrenal masses, adrenalectomy was conducted on his right adrenal gland and pathological examination revealed adrenal nodular hyperplasia (Fig. 1). One week after surgery, his serum and urinary free cortisol levels reduced significantly but remained at high levels. During the follow-up, the CS symptoms did not improve. Six months later, the patient was hospitalized and underwent subtotal left adrenalectomy. His blood pressure, CS symptoms and comorbidities including backache and muscle weakness improved thereafter.
Results of imaging and histopathology. A and B Adrenal computed tomography scan revealed bilateral adrenal masses (right: 7.2 × 5.2 × 5.1 cm, left: 6.9 × 4.5 × 3.2 cm, indicated by red arrows). C (X100, OLYMPUS microscope, OLYMPUS BX53) and D (X400, OLYMPUS microscope, OLYMPUS BX53) Hematoxylin and eosin staining of the adrenal tissue revealed nodular hyperplasia
Identification of the mutations
Whole exome sequencing (WES) was conducted on peripheral blood leukocytes and bilateral adrenal tumor tissues. A heterozygous germline mutation in ARMC5 (c. 1855C > T, p. R619*) was identified with 23 and 27 somatic mutations in his right and left adrenal, respectively (Table 3, Supplementary Table 1 and supplementary Table 2). Of note, three ARMC5 somatic mutations were detected in the right adrenal lesion, and two ARMC5 somatic mutations were found in the left adrenal gland (Table 3). Among them, four somatic mutations (p.A525_P536del, p.G99Efs*38, p.S95*, p.E848*) have not been previously reported in public databases and were predicted probably damaging by in silico models (MutationTaster and SIFT). One of them were long InDel and another three were substitutions leading to nonsense mutations. These novel mutations contributed to the mutation landscape of ARMC5 (Fig. 2) [8]. None of his family members had any history of endocrine disease and all of them refused to have their genes tested.
Discussion and conclusions
PBMAH is known as an autosomal positive disease characterized with multiple nodules in bilateral adrenal like “a bunch of grapes appearance” [9]. We report here a PBMAH patient presented with severe CS symptoms including moon face, centripetal obesity, purple striae of abdomen, limb edema, and muscle weakness, as well as its classical metabolic and bone complications like hypertension and osteoporosis. This patient carries one germline and 5 somatic pathogenic mutations in ARMC5. Of note, four of these somatic ARMC5 mutations have not been identified previously. AVS confirmed that the larger mass in his right adrenal produced more cortisol. One side of total followed by contralateral subtotal adrenalectomy appeared to work well for this particular patient.
The diagnosis of PBMAH in our case mainly depend on following four criteria [10]: (1) typical clinical manifestations and Cushing syndromes: central obesity, hypertension, purple striae, osteoporosis, and etc.; (2) abnormal laboratory tests, such as increased plasma cortisol levels and UFC, cortisol rhythm disorder, ACTH inhibition; (3) adrenal CT: multiple nodules in bilateral adrenal cortex (larger than 10 mm); and (4) pathology shows adrenal nodular hyperplasia. PBMAH has been considered as “ACTH-independent macronodular adrenal hyperplasia (AIMAH)” until Louiset et al. showed that local ACTH paracrine production by steroidogenic cells could lead to cortisol excess [11]. Previous studies indicate that plasma ACTH is suppressed in PBMAH patients, which could be used to discriminate PBMAH from ACTH-dependent CS, such as Cushing disease, ectopic secretion of ACTH [12, 13]. However, PBMAH needs to be discriminated from other ACTH-independent diseases, especially primary pigmented nodular adrenal disease (PPNAD) which could also present with CS and bilateral adrenal lesions. Imaging characteristics of PPNAD are bilateral adrenal micronodules with normal adrenal weight and size [3]; and pathological result often reveals pigmented nodules in PPNAD [14]. About 88% PPNAD are the endocrine manifestation of Carney complex (CNC) characterized by multiple endocrine and nonendocrine neoplasms and spotty skin pigmentation [15].
PBMAH is a highly heterogeneous disease with various degrees of cortisol secretion and CS. Most of PBMAH patients are not identified until overt CS occurs, patients with subclinical CS are hardly noticed unless the small enlargement of the adrenal is incidentally discovered [16]. Therefore, the phenotypes of PBMAH patients are diverse. Previous studies have identified the genotype-phenotype correlation in PBMAH patients [16]. Patients with mutant ARMC5 were diagnosed earlier with PBMAH with higher prevalence of overt CS and hypertension [16]. This patient carrying ARMC5 mutations presented with severe CS and classical metabolic and bone complications such as hypertension and osteoporosis also support the notion that PBMAH patients with ARMC5 mutations could have severe phenotypes. Previous studies have shown that the sizes of the adrenal masses are associated with the levels of cortisol and severity of Cushing syndrome [17, 18]. The large enough adrenal volume is needed to cause hypercortisolism, evidenced by that Cushing syndrome is usually observed in PBMAH patients with large adrenal masses [19]. Furthermore, ages of the PBMAH patients with ARMC5 mutations are correlated with cortisol hypersecretion [20].
ARMC5 is located at 16p11.2 with six exons and more than 80 mutations have been reported but without any identified hot spot [2]. ARMC5 mutations occur in up to 55% of operated PBMAH patients [16, 21]. In this report, we find our PBMAH patient carries a heterozygous germline mutation in ARMC5 (c.1855C > T) resulting in a premature stop codon (p. R619*) which has been previously reported as a frequently mutation in Japanese patients with PBMAH [1, 20]. Based on Knudson’s ‘two-hit’ theory of a tumor suppressor, a second somatic ARMC5 mutation would be needed in the background of the germline mutation [1]. Indeed, we found three and two (4 of them were previously unreported) side-specific ARMC5 somatic mutations in his right and left adrenal tumors, respectively. Previous study has revealed these mutations could be nodule-specific [1, 22], which might be associated with multiple individual macronodule formations although the mechanisms in these mutation-mediated PBMAH need to be further studies.
For patients with no evidence of cortisol excess at diagnosis, active surveillance is proposed with annual clinical and biochemical assessment. On the other hand, for patients with overt CS or clinical consequences of hypercortisolism (i.e., diabetes, hypertension, and bone fragility), a surgical or medical treatment should be taken into consideration. Steroid synthesis inhibitors including metyrapone and ketoconazole [23], mifepristone and glucocorticoid receptor antagonist [24], and mitotane [25] were the suggested medical treatment. Bilateral total adrenalectomy has been the mainstay of the treatment for PBMAH with CS [26], but life-long corticosteroid replacement is needed and associated health care burden including risk of adrenal crisis are unavoidable. Unilateral adrenalectomy has less complications [27] although recurrence of hypercortisolism exists [19, 28]. Recent studies suggest that total adrenalectomy of the larger adrenal and subtotal adrenalectomy of the contralateral adrenal (adrenal-sparing surgery) could be the better choice [29]. However, it remains challenging to decide which adrenal should be removed first and to what extent the subtotal adrenalectomy should be conducted on the contralateral adrenal. Our AVS results confirmed that the right adrenal with larger sizes of nodules produced more cortisol and thus the right adrenalectomy was initially performed.
In conclusion, PBMAH patient with ARMC5 mutations has severe CS symptoms and serious complications. Somatic ARMC5 mutations might be nodule-specific. AVS combined with the sizes of the nodules is helpful in identifying the dominant side of bilateral adrenal lesion of cortisol secretion.
Availability of data and materials
The raw sequencing data from this study have been deposited in the Genome Sequence Archive (GSA) with the project number of PRJCA012523.
Abbreviations
- ARMC5:
-
Armadillo repeat containing 5
- PBMAH:
-
Primary bilateral macronodular adrenal hyperplasia
- CS:
-
Cushing syndrome
- SCS:
-
Subclinical Cushing syndrome
- CT:
-
Computed tomography
- WES:
-
Whole-exome sequencing
- AVS:
-
Adrenal venous sampling
- LD-DST:
-
Low-dosed dexamethasone suppression test
- HD-DST:
-
High-dosed dexamethasone suppression test
- PPNAD:
-
Primary pigmented nodular adrenal disease
- AIMAH:
-
ACTH-independent macronodular adrenal hyperplasia
References
Assie G, Libe R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome. N Engl J Med. 2013;369(22):2105–14.
Bouys L, Chiodini I, Arlt W, Reincke M, Bertherat J. Update on primary bilateral macronodular adrenal hyperplasia (PBMAH). Endocrine. 2021;71(3):595–603.
Chevalier B, Vantyghem MC, Espiard S. Bilateral adrenal hyperplasia: pathogenesis and treatment. Biomedicines. 2021;9(10):1397.
Faucz FR, Zilbermint M, Lodish MB, Szarek E, Trivellin G, Sinaii N, et al. Macronodular adrenal hyperplasia due to mutations in an armadillo repeat containing 5 (ARMC5) gene: a clinical and genetic investigation. J Clin Endocrinol Metab. 2014;99(6):E1113–9.
Bouys L, Vaczlavik A, Jouinot A, Vaduva P, Espiard S, Assie G, et al. Identification of predictive criteria for pathogenic variants of primary bilateral macronodular adrenal hyperplasia (PBMAH) gene ARMC5 in 352 unselected patients. Eur J Endocrinol. 2022;187(1):123–34.
Albiger NM, Regazzo D, Rubin B, Ferrara AM, Rizzati S, Taschin E, et al. A multicenter experience on the prevalence of ARMC5 mutations in patients with primary bilateral macronodular adrenal hyperplasia: from genetic characterization to clinical phenotype. Endocrine. 2017;55(3):959–68.
Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, et al. Subtyping of primary Aldosteronism in the AVIS-2 study: assessment of selectivity and lateralization. J Clin Endocrinol Metab. 2020;105(6):2042–52.
Zhou ZJ, Qiu Y, Pu Y, Huang X, Ge XY. BioAider: an efficient tool for viral genome analysis and its application in tracing SARS-CoV-2 transmission. Sustain Cities Soc. 2020;63:102466.
Verma A, Mohan S, Gupta A. ACTH-independent macronodular adrenal hyperplasia: imaging findings of a rare condition : a case report. Abdom Imaging. 2008;33(2):225–9.
Zhang F, Lin X, Yu X. Primary macronodular adrenal hyperplasia (PMAH) can be generated by a new ARMC5 germline variant (c.52C>T (p.Gln18X)). Endocr J. 2020;67(12):1179–86.
Louiset E, Lefebvre H. Intraadrenal corticotropin in bilateral macronodular adrenal hyperplasia. N Engl J Med. 2014;370(11):1071–2.
Sohaib SA, Hanson JA, Newell-Price JD, Trainer PJ, Monson JP, Grossman AB, et al. CT appearance of the adrenal glands in adrenocorticotrophic hormone-dependent Cushing's syndrome. AJR Am J Roentgenol. 1999;172(4):997–1002.
Pivonello R, De Martino MC, De Leo M, Lombardi G, Colao A. Cushing's syndrome. Endocrinol Metab Clin N Am. 2008;37(1):135–49. ix
Stratakis CA. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin- independent Cushing syndrome). Endocr Dev. 2008;13:117–32.
Liu Q, Tong D, Liu G, Yi Y, Zhang D, Zhang J, et al. Carney complex with PRKAR1A gene mutation: a case report and literature review. Medicine (Baltimore). 2017;96(50):e8999.
Espiard S, Drougat L, Libe R, Assie G, Perlemoine K, Guignat L, et al. ARMC5 mutations in a large cohort of primary macronodular adrenal hyperplasia: clinical and functional consequences. J Clin Endocrinol Metab. 2015;100(6):E926–35.
Drougat L, Espiard S, Bertherat J. Genetics of primary bilateral macronodular adrenal hyperplasia: a model for early diagnosis of Cushing's syndrome?. Eur J Endocrinol. 2015;173(4):M121-131.
Rubinstein G, Osswald A, Braun LT, Vogel F, Kroiss M, Pilz S, et al. The role of adrenal venous sampling (AVS) in primary bilateral macronodular adrenocortical hyperplasia (PBMAH): a study of 16 patients. Endocrine. 2022;76(2):434–45.
Debillon E, Velayoudom-Cephise FL, Salenave S, Caron P, Chaffanjon P, Wagner T, et al. Unilateral adrenalectomy as a first-line treatment of Cushing's syndrome in patients with primary bilateral macronodular adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(12):4417–24.
Kyo C, Usui T, Kosugi R, Torii M, Yonemoto T, Ogawa T, et al. ARMC5 alterations in primary macronodular adrenal hyperplasia (PMAH) and the clinical state of variant carriers. J Endocr Soc. 2019;3(10):1837–46.
Vassiliadi DA, Tsagarakis S. Diagnosis and management of primary bilateral macronodular adrenal hyperplasia. Endocr Relat Cancer. 2019;26(10):R567–81.
Correa R, Zilbermint M, Berthon A, Espiard S, Batsis M, Papadakis GZ, et al. The ARMC5 gene shows extensive genetic variance in primary macronodular adrenocortical hyperplasia. Eur J Endocrinol. 2015;173(4):435–40.
Debono M, Harrison RF, Chadarevian R, Gueroult C, Abitbol JL, Newell-Price J. Resetting the abnormal circadian cortisol rhythm in adrenal Incidentaloma patients with mild autonomous cortisol secretion. J Clin Endocrinol Metab. 2017;102(9):3461–9.
Cohan P, East HE, Galati SJ, Mercado JU, Lim PJ, Lamerson M, et al. Mifepristone treatment in four cases of primary bilateral macronodular adrenal hyperplasia (BMAH). J Clin Endocrinol Metab. 2019;104(12):6279–90.
Nagai M, Narita I, Omori K, Komura S, Arakawa M. Adrenocorticotropic hormone-independent bilateral adrenocortical macronodular hyperplasia treated with mitotane. Intern Med. 1999;38(12):969–73.
Guerin C, Taieb D, Treglia G, Brue T, Lacroix A, Sebag F, et al. Bilateral adrenalectomy in the 21st century: when to use it for hypercortisolism? Endocr Relat Cancer. 2016;23(2):R131–42.
Powell AC, Stratakis CA, Patronas NJ, Steinberg SM, Batista D, Alexander HR, et al. Operative management of Cushing syndrome secondary to micronodular adrenal hyperplasia. Surgery. 2008;143(6):750–8.
Osswald A, Quinkler M, Di Dalmazi G, Deutschbein T, Rubinstein G, Ritzel K, et al. Long-term outcome of primary bilateral macronodular adrenocortical hyperplasia after unilateral adrenalectomy. J Clin Endocrinol Metab. 2019;104(7):2985–93.
Yoshiaki Tanno F, Srougi V, Almeida MQ, Ide Yamauchi F, Morbeck Almeida Coelho F, Nishi MY, et al. A new insight into the surgical treatment of primary macronodular adrenal hyperplasia. J Endocr Soc. 2020;4(8):bvaa083.
Acknowledgments
The authors thank the patient in this study for his collaboration and the Geneplus-Beijing for conducting the sequencing.
Funding
This work was supported by University Research Project of Army Medical University (2018XLC3073 for Jun Zhang; 2018XLC1014 and 2019CXLCB006 for Jun Jiang). These fundings supported this study in design of the study and collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Contributions
Study design were conducted by WL, JJ, QL, and JZ. Analysis of data were performed by PT,SP, XY, SW, YZ, and GL, and pathology studies by JX and YH. DZ, QL, and PT wrote the manuscript which was revised and approved by all authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures involving human participants were carried out in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was reviewed and approved by the ethics committee in the Daping Hospital (approval number 2018–93). The patient provided their written informed consent to participate in this study.
Consent for publication
Written informed consent for publication of identifying images or other personal or clinical details was obtained from all of the participants.
Competing interests
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Identified other 20 somatic single nucleotide variants (SNVs)/insertion-deletion (indel) mutations in the right adrenal mass.
Additional file 2: Supplementary Table 2.
Identified other 25 somatic single nucleotide variants (SNVs)/insertion-deletion (indel) mutations in the left adrenal mass.
Additional file 3.
CARE Checklist of information to include when writing a case report.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tang, P., Zhang, J., Peng, S. et al. Primary bilateral macronodular adrenocortical hyperplasia (PBMAH) patient with ARMC5 mutations. BMC Endocr Disord 23, 77 (2023). https://doi.org/10.1186/s12902-023-01324-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-023-01324-3