Abstract
We investigated the prevalence and clinical metabolic characteristics of lean nonalcoholic fatty liver disease (NAFLD) in an elderly Chinese population and assessed the relevance of lipid markers and genetic variation. All 5,338 community subjects underwent detailed clinical and laboratory examinations and were divided into three groups: lean (Body mass index (BMI) < 23 kg/m2, n = 2,012), overweight (BMI = 23–24.9 kg/m2, n = 1,354), and obese (BMI ≥ 25 kg/m2, n = 1,972). Single nucleotide polymorphisms were selected based on those reported in previous NAFLD or obesity genome-wide association studies. The frequencies of alleles and genotypes were calculated and statistically analyzed with Pearson’s χ2 tests. One-way ANCOVA was used to test the association between positive SNPs and metabolic parameters in lean NAFLD individuals. Our results showed that the C allele frequency of rs2279026, the G allele of rs2279028, the C allele of rs780093, and the C allele frequency of rs1260326 were higher in obese NAFLD than in lean NAFLD (P < 0.05). In addition, we observed an association between the CC of rs1421085, TT of rs3751812, AA of rs8050136, and AA of rs9939609 genotypes in the FTO gene and low-density lipoprotein levels (P < 0.05). In conclusion, our findings provide a unique perspective on the prevalence, genetic characteristics, and metabolic profile of NAFLD in older lean individuals in China. This is the first study to examine the association between genetic variants in the FTO, TFAP2B and GCKR genes and NAFLD in a cohort of lean individuals.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most widespread chronic liver diseases worldwide [1], the global prevalence of NAFLD is 25.24% [2]. Especially in China, the prevalence has reached 29.2% in 2019 [3]. NAFLD can range from simple steatosis to nonalcoholic steatohepatitis (NASH), which progresses to severely progressive liver cirrhosis and hepatocellular carcinoma [4]. NAFLD is strongly related to obesity, type 2 diabetes mellitus (T2DM), cardiovascular disease, dyslipidemia, and hypertension [5, 6]. Even though NAFLD often arises in the context of obesity, it also can be found in non-obese/lean subjects and presents with disease severity similar to that of obese NAFLD subjects [7]. Diagnosing NAFLD early is essential to halt the progression. Body mass index (BMI) is one of the etiological markers [8], however, it was found that using BMI to determine excess adiposity has limited sensitivity due to its inability to distinguish between fat and lean mass [9]. We attempted to explore a more precise and plausible metric that could be widely used for the diagnosis and assessment of disease for lean NAFLD.
Genetic variation contributes to differences in phenotypic traits in humans [10]. The pathogenesis underlying NAFLD progression and occurrence can be understood better by studying genetic factors that lead to NAFLD [11]. By interlocking and mutually reinforcing pathways, abnormal lipid metabolism, insulin resistance, and oxidative stress, along with inflammation, contribute to NAFLD [12]. As is well known, alterations in lipid metabolism are fundamental to the development and progression of NAFLD [13]. Hitherto, the best-known NAFLD inherited components are represented by single nucleotide polymorphisms (SNPs) in genes regulating hepatic lipid accumulation and metabolism, among which FTO alpha-ketoglutarate dependent dioxygenase (FTO) [14], transcription factor AP-2 beta (TFAP2B) [15], TBC1 domain family member 1 (TBC1D1) [16], glucokinase regulator (GCKR) [17], and potassium inwardly-rectifying channel subfamily J member 11 (KCNJ11) [18] have been evaluated as genetic determinants of NAFLD. In obese and lean individuals, nonsynonymous variants of the FTO gene are found identically, suggesting that the FTO protein itself might not be the sole explanation for the association of the FTO locus with obesity in humans [19, 20].
There is also the possibility of NAFLD occurring in lean individuals with genetic backgrounds, contributing to similar liver characteristics. The interaction of BMI and genetic factors in Asians warrants further investigation. We hope to discover the specific genetic variant of lean NAFLD by studying the metabolic and genetic variation characteristics of NAFLD so that we can better explain the pathogenesis of lean NAFLD. Therefore, we aimed to investigate the interaction of BMI with several known or potential genetic risk variables in a general population cohort regarding susceptibility and lipid metabolism in NAFLD and lean NAFLD. This study provides complete information on genetic polymorphism in the general Asian population and elucidates the genetic risk of NAFLD in lean individuals.
Materials and methods
Subjects
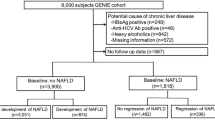
This was a post-doc analysis of a cross-sectional population study conducted in Shanghai, China. We recruited 5387 residents from the Zhanjiang community of Shanghai aged ≥ 60 years and older between April and July 2017 for our study (Fig. 1). The study followed the Helsinki Declaration. A standard protocol has been developed by the Shanghai Innovation Center of Traditional Chinese Medicine Health Service and approved by the Ethics Committee of the Shanghai University of Traditional Chinese Medicine. All study participants provided informed consent. Participants with age ≥ 60 years, who live in Shanghai, can complete measurements and informed consent was included in the inclusion criteria. This study excluded participants with mental disorders, malignant tumors, or incomplete medical records. During the investigation, 49 subjects were excluded, resulting in a total of 5338 Chinese elderly subjects with complete data (2470 non-NAFLD and 2868 NAFLD).
In 2017, 1449 participants from the community cohort database who meet the following criteria were genotyped: permanent residents of Zhangjiang area of Pudong District, Shanghai; Chinese Han population without blood relations; and all medical examination, biochemical, and specimen collection information were complete and accurate. We excluded subjects who abused alcohol (< 140 g/week in males and < 70 g/week in females), had hepatitis B or C or had drug-induced or autoimmune liver disease history. In the genotype population cohort, 230 subjects were excluded, including missing BMI data and those < 60 years of age. The diagnosis of NAFLD was made based on 2010 guidelines from the Chinese Medical Association for its management [21].
Clinical data collection
Two experienced radiologists evaluated fatty liver using a color ultrasound system [22]. Based on the criteria above, with NAFLD with a median (interquartile range) age of 69.02 (62.10–75.94) healthy controls from the same geographical area with a median age of 69.39 (62.03–76.75) were included in this study.
Questionnaires were used to collect information such as age, gender, alcohol consumption, current smoking, and medical history. We measured height and body weight using electronic measurement instruments (Sheng-yuan, Zhengzhou, China) wearing light clothing without hats or shoes, to the nearest 0.1 cm and 0.1 kg, respectively. Based on the BMI (body weight in kilograms divided by height in meters squared (kg/m2)), three categories were established: lean (BMI < 23 kg/m2), overweight (BMI between 23 and 24.9 kg/m2), and obese (BMI ≥ 25 kg/m2) [23] patients with NAFLD. Electronic sphygmomanometers were used to measure blood pressure (Bio-space, Cheonan, South Korea). We collected blood samples from the antecubital vein after fasting overnight in the morning. The following parameters were measured using an automatic biochemistry analyzer (Hitachi, Tokyo, Japan): fasting glucose, hemoglobin, platelets, ureophils, creatinine, uric acid, total cholesterol (TC), total bilirubin (TB), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), alanine transaminase (ALT), and aspartate transaminase (AST).
Extracting DNA, selecting SNPs, and genotyping
The genomic DNA was extracted from peripheral venous blood using the standard phenol–chloroform method for genotyping by collecting 5 mL of blood from each subject. The SNPs selected are based on positive reports of NAFLD or obesity from previous genome-wide association studies (GWAS) [14,15,16,17,18,19,20, 24, 25]. In this study, we examined the role of genes FTO, TFAP2B, TBC1D1, GCKR, and KCNJ11 in NAFLD in Chinese Han individuals (Table 1). Twelve SNPs (rs1421085, rs3751812, rs8050136, and rs9939609 in the FTO gene, rs2206277 in the TFAP2B gene, rs2279027, rs2279026, and rs2279028 in the TBC1D1 gene, rs780093, rs780094, and rs1260326 in the GCKR gene, and rs5215 in the KCNJ11 gene) were genotyped in this study. With the Mass ARRAY® Analyzer 4 platform (Sequenom, San Diego, CA), all 12 of these SNPs have successfully been genotyped by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry. Probes and primers were designed using Mysequenom online software along with Assay Design Suite v2.0. Standard polymerase chain reaction (PCR) was carried out in a volume of 5 μL containing 10 ng of genomic DNA. The primers and conditions of the PCR reactions are available upon request.
Statistical analysis
All statistical tests were conducted using SPSS Statistics (IBM, version 26.0, Armonk, NY, USA). The continuous variables were expressed as mean ± standard deviation and compared using the Mann–Whitney U test or independent sample t-test as appropriate. Categorical variables were compared using the χ2 test. We tested the association between positive SNPs and metabolic parameters in lean NAFLD individuals using a one-way ANCOVA adjusted for age. Analyzing the distributions of alleles and genotypes was performed using SHEsis (http://analysis.bio-x.cn/my Analysis.php) (Bio-X Institutes, Shanghai, China) [26]. Categorical variables were expressed as counts or percentages and compared using Pearson’s χ2 tests. Hardy–Weinberg equilibrium (HWE) was assessed by Pearson’s χ2 tests. As well as odds ratios, 95% confidence intervals (CIs) were calculated. There was a statistical significance of P < 0.05.
Results
Demographics and clinical metabolic phenotypes
The overall clinical and laboratory characteristics of the 2470 non-NAFLD subjects and 2868 NAFLD subjects are listed in Table 2. The mean age of the subjects was 69.19 ± 7.13 years, and 2400 (45.0%) were male (Table 2). A total of 1324 (24.8%), 2861 (53.6%), 833(15.6%), 892 (16.7%), and 281 (5.3%) subjects had metabolic syndrome, hypertension, diabetes, cardiopathy, and hyperlipidemia, respectively. The subjects with NAFLD had a higher prevalence of metabolic syndrome, hypertension, and diabetes compared with the subjects without NAFLD (P < 0.001). A total of 2012 (37.7%) subjects had BMI < 23 kg/m2, 1354 (25.4%) subjects with BMI ranging from 23 to 24.9 kg/m2 and 1972 (36.9%) subjects with BMI ≥ 25.0 kg/m2. The prevalence of NAFLD was 19.2% in lean subjects, 28.6% in overweight subjects, and 52.2% in obese subjects (P < 0.001). As shown in Table 2, the subjects with NAFLD tended to have significantly higher BMI, waist circumference (WC), waist-to-hip ratio, systolic blood pressure, and diastolic blood pressure compared with the subjects without NAFLD (P < 0.001). Patients with NAFLD were significantly taller and presented higher mean values of ALT, fasting glucose, hemoglobin, platelet, TC, LDL, TG, and uric acid, and lower mean values of creatinine and HDL than non-NAFLD.
Genotyping of NAFLD risk loci
To determine if the genetic underpinning of NAFLD differs from the genetic underpinning of non-NAFLD subjects, we analyzed SNPs that have previously been identified as related to NAFLD and obesity in GWAS [12,13,14,15,16,17,18, 22, 23]. In the genotyped population cohort, there were eventually 750 subjects with NAFLD and 469 subjects with non-NAFLD included in the analysis. For the rs1421085 (73.9% for TT, 24.6% for CT and 1.3% for CC), rs3751812 (73.5% for GG, 25.1% for GT and 1.3% for TT), rs8050136 (74.1% for CC, 24.4% for CA and 1.3% for AA), rs9939609 (73.4% for TT, 1.3% for AA and 25.2% for AT), rs2206277 (35.8% for CT, 8.0% for TT and 56.1% for CC) and rs1260326 (18.6% for CC, 29.9% for TT and 51.4% for CT), all of these genotype groups showed significant prevalence in NAFLD subjects (P < 0.05) (See Supplementary file 1).
The allele frequencies of rs1421085, rs3751812, rs8050136, rs9939609, and rs2206277 were significantly different between NAFLD and non-NAFLD (P < 0.05). The C allele frequency of rs1421085 was remarkably higher in NAFLD individuals (OR = 1.407; 95%CI = 1.083–1.828; P = 0.010). The occurrence of T allele of rs3751812 was significantly increased in NAFLD group (OR = 1.443; 95% CI = 1.114–1.869; P = 0.005). In rs8050136, the A allele frequency was remarkably higher in NAFLD (OR = 1.430; 95% CI = 1.099–1.861; P = 0.007). And the A allele frequency of rs9939609 was also significantly increased in NAFLD (OR = 1.429; 95% CI = 1.105–1.849; P = 0.006). The T allele frequency of rs2206277 was remarkably higher in NAFLD individuals (OR = 1.305; 95%CI = 1.074–1.586; P = 0.007) (See Supplementary file 1).
Genotyping of lean NAFLD risk loci
We separated the NAFLD population into three categories for subgroup analysis: lean NAFLD (n = 106), overweight NAFLD (n = 184), and obese NAFLD (n = 460). Importantly, the allele frequencies of rs2206277, rs2279026, rs2279028, rs780093, rs1260326, and rs5215 were different between overweight/ obese NAFLD and lean NAFLD (P < 0.05). The C allele frequency of rs2279026 (OR = 1.501; 95%CI = 1.074–2.098; P = 0.016), the G allele frequency of rs2279028 (OR = 1.516; 95%CI = 1.084–2.119; P = 0.014), the C allele frequency of rs780093 (OR = 1.381; 95%CI = 1.013–1.883; P = 0.040), and the C allele frequency of rs1260326 (OR = 1.376; 95%CI = 1.011–1.874; P = 0.041) were higher in obese NAFLD than lean subjects presenting NAFLD (See Supplementary file 2). When compared to lean NAFLD, the occurrence of C allele of rs780093 was significantly increased in overweight subjects presenting NAFLD (OR = 1.425; 95%CI = 1.006–2.019; P = 0.045) (See Supplementary file 2). Compared to lean NAFLD subjects, the T allele frequency of rs2206277 and the C allele frequency of rs5215 were both significantly lower in obese subjects presenting NAFLD (OR = 0.705, 95%CI = 0.521–0.953; P = 0.022; OR = 0.671; 95%CI = 0.484–0.931; P = 0.016) (See Supplementary file 2). There was no genotyping difference between lean NAFLD and overweight/obese NAFLD in the HWE paradigm.
Association of genotypes and the phenotype in lean subjects
TFAP2B rs2206277 was associated with ALT in overweight subjects (P = 0.021), however, the difference was not significant between the lean and the obese (Fig. 2A). The association of GCKR rs1260326 genotype with AST and TG was significant only in obese subjects (Fig. 2B, F). Additionally, we observed that GCKR rs1260326 was associated with TB in both lean and overweight subjects (P < 0.05, Fig. 2C). FTO rs1421085 was associated with TB in obese subjects (P = 0.035, Fig. 2D). The relationship between TFAP2B rs2206277 genotype and TB was significant in the lean group (P = 0.033, Fig. 2E). The association between four SNPs, i.e., rs1421085, rs3751812, rs8050136, and rs9939609 in the FTO gene and LDL was significant in lean subjects but not in overweight and obese subjects (P < 0.05, Fig. 2G-J). There was no significant association between rs1421085, rs3751812, rs8050136, and rs9939609 and TFAP2B rs2206277 genotypes and TG. In addition, no significant association was found between the genotypes of six SNPs and TC/HDL (See Supplementary file 3). There was no significant association between the FTO rs1421085, rs3751812, rs8050136, and rs9939609, as well as the TFAP2B rs2206277 genotype and TG. In addition, no significant association between the genotypes of the six SNPs with TC/HDL was found (See Supplementary file 3).
Association between clinical metabolic phenotypes with the FTO, TFAP2B, and GCKR genotype, stratified by BMI. Abbreviations: LN, Lean NAFLD; OWN, Overweight NAFLD; ON, Obese NAFLD; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; ALT, alanine aminotransferase; AST, aspartate aminotransferase. P-value: Adjusted for age in ANCOVA. P < 0.05 was identified as a statistical significance
Discussion
In the present cross-sectional study, we found that the prevalence of metabolic syndrome, hypertension, and diabetes was significantly higher in NAFLD subjects than in non-NAFLD, and NAFLD subjects had a significantly higher BMI, WC, waist-to-hip ratio, blood pressure, hemoglobin, platelets, ALT, fasting glucose, TC, LDL, TG, and uric acid, and lower creatinine and HDL. These results are consistent with other cohorts’ studies [27]. It suggests that inflammatory and lipid-related metabolic features are more pronounced in older adults who have developed NAFLD and, more importantly, that clinicians should pay more attention to the potentially controversial role that metabolic-related comorbidities may have in NAFLD disease.
To investigate the genetic basis of NAFLD versus non-NAFLD subjects, we found that genotypes and alleles at the rs1421085, rs8050136, rs3751812, rs9939609 for FTO, rs2206277 for TFAP2B, and rs1260326 for GCKR loci were associated with increased risk of NAFLD. This result has been partially confirmed by the previous study from our group [28]. Several studies have reported that the FTO gene is critical in regulating body weight and fat mass, and plays a role in the energetic metabolism and regulation of the organism’s homeostasis [29]. In addition, we focused on the association of TFAP2B genotype with genetic susceptibility to NAFLD. The prevalence of NAFLD has been proven to be involved in obesity, T2DM, hyperlipidemia, and insulin resistance [30]. The metabolic syndrome is as well considered to be a risk factor for NAFLD [31]. Based on genome-wide association analysis data, genetic variants in FTO and TFAP2B, previously known as obesity susceptibility loci, impinge independently on the risk of metabolic syndrome [31]. Studies from Asia have revealed that genetic predisposition to the FTO and TFAP2B genes contributed to a sex-dependent pattern of obesity-related traits and remarkable accumulation of abdominal fat [32, 33]. The transcription factor TFAP2B is involved in regulating adipocyte metabolism by stimulating glucose uptake and lipid accumulation while decreasing insulin sensitivity [34]. Reduced expression of TFAP2B may have a protective effect against diminished insulin sensitivity and central obesity-related complications such as T2DM and coronary artery disease [34, 35]. Notably, a single mutant genetic variant rs1260326 in the glucokinase regulatory protein gene GCKR is implicated in the stress index of insulin resistance [36]. A retrospective cohort study from Iranian adult subjects identified a strongly associated GCKR variant with the prevalence of metabolic syndrome [37], and genetic variants of GCKR have been demonstrated to be involved in hepatic redox and contribute causally to key metabolic traits and diseases [38]. As hepatocyte lipid deposition is the initiating step and hallmark of NAFLD etiopathology [39], all three genes are involved in lipid metabolic pathways, such as adipogenesis, which are intimately associated with insulin resistance and NAFLD, all these facts directly or indirectly supported our finding.
To present, the relevance of SNPs to NAFLD has not been addressed in Chinese lean adults. In our contextual research sample, 19.2% of the NAFLD population suffered from lean NAFLD. Globally, about 40% of the global NAFLD population belongs to the non-obese population, and nearly one-fifth falls into the lean individuals [40]. Lean NAFLD subjects may have a more severe histological phenotype, higher mortality, and morbidity, and an increased risk of developing metabolic disease and progressing to more severe liver disease compared to overweight and obese NAFLD subjects [41, 42]. Several clinical, genetic, and metabolic findings suggest that NAFLD in lean individuals appears to be particularly warranted. In this study, we found that C allele rs2279026 of the TBC1D1 gene, G allele rs2279028 of the TBC1D1 gene, C allele rs780093 of the GCKR gene, and C allele rs1260326 of the GCKR gene were associated with a decreased risk of lean NAFLD in Chinese elderly population, T allele rs2206277 of the TFAP2B and C allele rs5215 of the KCNJ11 were combined with an increased risk of lean NAFLD. Alternatively, in a subgroup analysis of the NAFLD population, we found that the C allele of rs2279026 of the TBC1D1 gene, the G allele of rs2279028 of the TBC1D1 gene, the C allele of rs780093 of the GCKR gene, and the C allele of rs1260326 of the GCKR gene were strongly associated with increased risk of NAFLD in obesity. The C allele of rs780093 of the GCKR gene was substantially more prevalent in overweight NAFLD. Genetic studies have shown that sterol regulatory protein binding factor (SREBF), transmembrane superfamily member 2 (TM6SF2), cholesteryl ester transfer protein, apolipoprotein C3 (APOC3), and patatin-like phospholipase domain-containing 3 (PNPLA3) have a promising contribution to the development and long-term prognosis of NAFLD in obese and lean individuals [43]. In a recently conducted investigation of risk variables for NAFLD in lean Chinese adults, investigators found no genotypic significant differences in common SNPs (sirtuin 1 (SIRT1) rs2273773, APOC3 rs2070666, PNPLA3 rs738409, PNPLA3 rs738408, PNPLA3 rs4823173, PNPLA3 rs2072906, angiotensin II receptor type 1 (AGTR1) rs5186, and AGTR1 rs440881) between lean subjects with and without NAFLD [44]. There is a similarity between such results and ours. Finally, our study complements the contribution of genetic factors at select loci in NAFLD in Chinese lean individuals, even due to the limited number of SNPs we measured.
Lean NAFLD subjects may have clinically similar metabolic dysfunction to obese or overweight individuals [45], even if they are at a much higher risk of developing metabolic dysfunction [46]. The pathogenesis of NAFLD in lean and obese individuals is not exclusively congruent. Regarding the majority of metabolic parameters, such as ALT, AST, TC, TB, TG, HDL, and LDL, there appeared to be discrepancies between the lean NAFLD subjects and the overweight/obese ones [47]. We observed for the first time that GCKR genotype rs1260326 for CC and TFAP2B genotype rs2206277 for CT were associated with TB in lean subjects, in which addition we found that GCKR genotype rs1260326 for TT was associated with TB in overweight recipients and FTO rs1421085 for CC was associated with TB in obese participants. We first observed that GCKR genotype rs1260326 for CC and TFAP2B genotype rs2206277 for CT were associated with TB in lean subjects, in addition we also found that GCKR genotype rs1260326 for TT was associated with TB in overweight subjects and FTO rs1421085 for CC was associated with TB in obese subjects. Studies on the correlation between genotype and TB are currently poorly reported. Some investigators have found that the UGT1A1 gene is a dominant genetic determinant of bilirubin concentration and that the UGT1A1*28 genotype is associated with an increased likelihood of hyperbilirubinemia and shows different features of association among different races [48]. We found that FTO, TFAP2B, and GCKR genotype variants interacted with certain metabolic profiles in lean NAFLD subjects. The FTO genotype of rs1421085 for CC, rs3751812 for TT, rs8050136 for AA, and rs9939609 for AA were associated with LDL levels, but no association was found between these genotypes and TG, TC, and HDL. The association of FTO genotype variants with lipid-related metabolic parameters has been documented, but mainly concerning overweight or obesity exposure, however, we must not discount that lean is also susceptible to lipid metabolic disorders [49], and we conducted the first analysis of the mutual interaction of FTO genotype variants with lean NAFLD and definite metabolic distinctions. A case–control analysis study of 612 Pakistani subjects found that the interaction between the CT genotype of rs1421085 and overweight/obesity affects TG and LDL independent of age and sex [50]. From another report, FTO gene expression was shown to be associated with LDL, and fasting glucose, but not TG, TC, and HDL [51]. We are also interested in an investigation of the association of the FTO rs9939609 polymorphism with lipid profiles in Iranian women, who noted that lower levels of HDL were observed in the AT/AA genotype compared to the TT wild-type genotype of the FTO rs9939609 polymorphism, with adjustments for age, BMI and physical activity that did not alter the results [52]. A cohort study of Polish Caucasians showed similar results, with GG genotype (rs3751812) and CC genotype (rs8050136) carriers having significantly higher TC and LDL levels when stratified into groups above the median fiber intake [53]. Given that our results revealed unique genetic factors and metabolic disorders in lean individuals with NAFLD, it would make sense to investigate this in Chinese subjects as well.
There are some limitations of this study. First, our diagnosis of NAFLD rested on ultrasound, and the assessment of the degree of visceral fat accumulation in the liver could not be ascertained. Despite invasive liver biopsy examinations may improve the precision and credibility of our data, but may not be appropriate for the large community cohort study. And ultrasonography is widely used in studies of large population cohorts and has some validity and feasibility. Second, insulin levels and insulin resistance were inadequately analyzed, even though diet and insulin resistance may be strongly associated with NAFLD in lean recipients. Notwithstanding the limitations, our findings provide critical insights into the morbidity, genetic characteristics, and metabolic facets of NAFLD in the Chinese elderly lean subjects. This is the first study to address the association of FTO, TFAP2B, and GCKR gene variants with NAFLD in a lean cohort.
Conclusions
In conclusion, our findings provide unique perspectives on the morbidity, genetic characteristics, and metabolic profile of NAFLD in the Chinese elderly lean individuals. This is the first study to investigate the association of FTO, TFAP2B, and GCKR gene variants with NAFLD in a lean cohort. Notably, we observed an association between the CC of rs1421085, TT of rs3751812, AA of rs8050136, and AA of rs9939609 genotypes in the FTO gene and LDL levels. Future studies will be required to fully elucidate the role of gene-environment interactions in NAFLD and pathogenesis with a multi-omics approach.
Availability of data and materials
The de-identified data set is available on request to the corresponding author.
Abbreviations
- AGTR1 :
-
Angiotensin II receptor type 1
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- APOC3 :
-
Apolipoprotein C3
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- FTO :
-
FTO alpha-ketoglutarate dependent dioxygenase
- GCKR :
-
Glucokinase regulator
- GWAS:
-
Genome-wide association studies
- HDL:
-
High-density lipoprotein
- HWE:
-
Hardy–Weinberg equilibrium
- KCNJ11 :
-
Potassium inwardly-rectifying channel subfamily J member 11
- LDL:
-
Low-density lipoprotein
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- OR:
-
Odds ratios
- PNPLA3 :
-
Patatin-like phospholipase domain-containing 3
- SIRT1 :
-
Sirtuin 1
- SNPs:
-
Single nucleotide polymorphisms
- SREBF :
-
Sterol regulatory protein binding factor
- TB:
-
Total bilirubin
- TC:
-
Total cholesterol
- TBC1D1 :
-
TBC1 domain family member 1
- T2DM:
-
Type 2 diabetes mellitus
- TFAP2B :
-
Transcription factor AP-2 beta
- TG:
-
Triglycerides
- TM6SF2 :
-
Transmembrane superfamily member 2
- WC:
-
Waist circumference
References
Younossi Z, Tacke F, Arrese M, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69(6):2672–82. https://doi.org/10.1002/hep.30251.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431.
Zhou F, Zhou J, Wang W, et al. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology. 2019;70(4):1119–33. https://doi.org/10.1002/hep.30702.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. https://doi.org/10.1002/hep.29367.
Patel YA, Gifford EJ, Glass LM, et al. Risk factors for biopsy-proven advanced non-alcoholic fatty liver disease in the Veterans Health Administration. Aliment Pharmacol Ther. 2018;47(2):268–78. https://doi.org/10.1111/apt.14411.
Saki S, Saki N, Poustchi H, Malekzadeh R. Assessment of Genetic Aspects of Non-alcoholic Fatty Liver and Premature Cardiovascular Events. Middle East J Dig Dis. 2020;12(2):65–88. https://doi.org/10.34172/mejdd.2020.166.
Leung JC, Loong TC, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65(1):54–64. https://doi.org/10.1002/hep.28697.
López-Hernández L, Martínez-Arnau FM, Pérez-Ros P, Drehmer E, Pablos A. Improved Nutritional Knowledge in the Obese Adult Population Modifies Eating Habits and Serum and Anthropometric Markers. Nutrients. 2020;12(11):3355. https://doi.org/10.3390/nu12113355.
Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond). 2010;34(5):791–9. https://doi.org/10.1038/ijo.2010.5.
Kato M, Nakamura Y, Tsunoda T. An algorithm for inferring complex haplotypes in a region of copy-number variation. Am J Hum Genet. 2008;83(2):157–69. https://doi.org/10.1016/j.ajhg.2008.06.021.
Kim U, Kim N, Shin HY. Modeling Non-Alcoholic Fatty Liver Disease (NAFLD) Using "Good-Fit" Genome-Editing Tools. Cells. 2020;9(12).https://doi.org/10.3390/cells9122572
Christensen K, Lawler T, Mares J. Dietary Carotenoids and Non-Alcoholic Fatty Liver Disease among US Adults, NHANES 2003–2014. Nutrients. 2019;11(5):1101. https://doi.org/10.3390/nu11051101.
Rives C, Fougerat A, Ellero-Simatos S, et al. Oxidative Stress in NAFLD: Role of Nutrients and Food Contaminants. Biomolecules. 2020;10(12). https://doi.org/10.3390/biom10121702
Mizuno TM. Fat Mass and Obesity Associated (FTO) Gene and Hepatic Glucose and Lipid Metabolism. Nutrients. 2018;10(11):1600. https://doi.org/10.3390/nu10111600.
Stocks T, Angquist L, Banasik K, et al. TFAP2B influences the effect of dietary fat on weight loss under energy restriction. PLoS ONE. 2012;7(8):e43212. https://doi.org/10.1371/journal.pone.0043212.
Chen ZY, Sun YT, Wang ZM, et al. Rab2A regulates the progression of nonalcoholic fatty liver disease downstream of AMPK-TBC1D1 axis by stabilizing PPARγ. PLoS Biol. 2022;20(1):e3001522. https://doi.org/10.1371/journal.pbio.3001522.
Sliz E, Sebert S, Würtz P, et al. NAFLD risk alleles in PNPLA3, TM6SF2, GCKR and LYPLAL1 show divergent metabolic effects. Hum Mol Genet. 2018;27(12):2214–23. https://doi.org/10.1093/hmg/ddy124.
Gallardo-Blanco HL, Villarreal-Perez JZ, Cerda-Flores RM, et al. Genetic variants in KCNJ11, TCF7L2 and HNF4A are associated with type 2 diabetes, BMI and dyslipidemia in families of Northeastern Mexico: A pilot study. Exp Ther Med. 2017;13(2):523–9. https://doi.org/10.3892/etm.2016.3990.
Meyre D, Proulx K, Kawagoe-Takaki H, et al. Prevalence of loss-of-function FTO mutations in lean and obese individuals. Diabetes. 2010;59(1):311–8. https://doi.org/10.2337/db09-0703.
Zheng Z, Hong L, Huang X, et al. Screening for coding variants in FTO and SH2B1 genes in Chinese patients with obesity. PLoS ONE. 2013;8(6):e67039. https://doi.org/10.1371/journal.pone.0067039.
Zeng MD, Fan JG, Lu LG, et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9(2):108–12. https://doi.org/10.1111/j.1751-2980.2008.00331.x.
Sanford NL, Walsh P, Matis C, Baddeley H, Powell LW. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology. 1985;89(1):186–91. https://doi.org/10.1016/0016-5085(85)90761-9.
Shiwaku K, Anuurad E, Enkhmaa B, Kitajima K, Yamane Y. Appropriate BMI for Asian populations. Lancet. 2004;363(9414):1077 https://doi.org/10.16/S0140-6736(04)15856-X.
Hu Y, Feng Y, Zhang L, et al. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m(6)A on lipogenic mRNAs. RNA Biol. 2020;17(7):930–42. https://doi.org/10.1080/15476286.2020.1736868.
Petit JM, Masson D, Guiu B, et al. GCKR polymorphism influences liver fat content in patients with type 2 diabetes. Acta Diabetol. 2016;53(2):237–42. https://doi.org/10.1007/s00592-015-0766-4.
Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15(2):97–8. https://doi.org/10.1038/sj.cr.7290272.
Cheng YM, Kao JH, Wang CC. The metabolic profiles and body composition of lean metabolic associated fatty liver disease. Hepatol Int. 2021;15(2):405–12. https://doi.org/10.1007/s12072-021-10147-0.
Gu Z, Bi Y, Yuan F, et al. FTO Polymorphisms are Associated with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Susceptibility in the Older Chinese Han Population. Clin Interv Aging. 2020;15:1333–41. https://doi.org/10.2147/cia.S254740.
Larder R, Cheung MK, Tung YC, Yeo GS, Coll AP. Where to go with FTO? Trends Endocrinol Metab. 2011;22(2):53–9. https://doi.org/10.1016/j.tem.2010.11.001.
Tanase DM, Gosav EM, Costea CF, et al. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J Diabetes Res. 2020;2020:3920196. https://doi.org/10.1155/2020/3920196.
Kraja AT, Chasman DI, North KE, et al. Pleiotropic genes for metabolic syndrome and inflammation. Mol Genet Metab. 2014;112(4):317–38. https://doi.org/10.1016/j.ymgme.2014.04.007.
Hotta K, Nakamura M, Nakamura T, et al. Polymorphisms in NRXN3, TFAP2B, MSRA, LYPLAL1, FTO and MC4R and their effect on visceral fat area in the Japanese population. J Hum Genet. 2010;55(11):738–42. https://doi.org/10.1038/jhg.2010.99.
Kong X, Xing X, Zhang X, Hong J, Yang W. Sexual Dimorphism of a Genetic Risk Score for Obesity and Related Traits among Chinese Patients with Type 2 Diabetes. Obes Facts. 2019;12(3):328–43. https://doi.org/10.1159/000500490.
Ugi S, Nishio Y, Yamamoto H, et al. Relation of the Expression of Transcriptional Factor TFAP2B to That of Adipokines in Subcutaneous and Omental Adipose Tissues. Obesity. 2010;18(7):1277–82. https://doi.org/10.1038/oby.2009.442.
Nordquist N, Göktürk C, Comasco E, et al. The transcription factor TFAP2B is associated with insulin resistance and adiposity in healthy adolescents. Obesity (Silver Spring). 2009;17(9):1762–7. https://doi.org/10.1038/oby.2009.83.
Würtz P, Mäkinen VP, Soininen P, et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes. 2012;61(6):1372–80. https://doi.org/10.2337/db11-1355.
Zahedi AS, Akbarzadeh M, Sedaghati-Khayat B, Seyedhamzehzadeh A, Daneshpour MS. GCKR common functional polymorphisms are associated with metabolic syndrome and its components: a 10-year retrospective cohort study in Iranian adults. Diabetol Metab Syndr. 2021;13(1):20. https://doi.org/10.1186/s13098-021-00637-4.
Goodman RP, Markhard AL, Shah H, et al. Hepatic NADH reductive stress underlies common variation in metabolic traits. Nature. 2020;583(7814):122–6. https://doi.org/10.1038/s41586-020-2337-2.
He J, Ding J, Lai Q, Wang X, Li A, Liu S. Irbesartan Ameliorates Lipid Deposition by Enhancing Autophagy via PKC/AMPK/ULK1 Axis in Free Fatty Acid Induced Hepatocytes. Front Physiol. 2019;10:681. https://doi.org/10.3389/fphys.2019.00681.
Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–52. https://doi.org/10.1016/s2468-1253(20)30077-7.
Denkmayr L, Feldman A, Stechemesser L, et al. Lean Patients with Non-Alcoholic Fatty Liver Disease Have a Severe Histological Phenotype Similar to Obese Patients. J Clin Med. 2018;7(12):562. https://doi.org/10.3390/jcm7120562.
Hu PF, Zeng X, Zou ZY, et al. The presence of NAFLD in nonobese subjects increased the risk of metabolic abnormalities than obese subjects without NAFLD: a population-based cross-sectional study. Hepatobiliary Surg Nutr. 2021;10(6):811–24. https://doi.org/10.21037/hbsn-20-263.
Ahadi M, Molooghi K, Masoudifar N, Namdar AB, Vossoughinia H, Farzanehfar M. A review of non-alcoholic fatty liver disease in non-obese and lean individuals. J Gastroenterol Hepatol. 2021;36(6):1497–507. https://doi.org/10.1111/jgh.15353.
Zeng J, Yang RX, Sun C, et al. Prevalence, clinical characteristics, risk factors, and indicators for lean Chinese adults with nonalcoholic fatty liver disease. World J Gastroenterol. 2020;26(15):1792–804. https://doi.org/10.3748/wjg.v26.i15.1792.
Alam S, Eslam M, Skm Hasan N, et al. Risk factors of nonalcoholic fatty liver disease in lean body mass population: A systematic review and meta-analysis. JGH Open. 2021;5(11):1236–49. https://doi.org/10.1002/jgh3.12658.
Semmler G, Wernly S, Bachmayer S, et al. Nonalcoholic Fatty Liver Disease in Lean Subjects: Associations With Metabolic Dysregulation and Cardiovascular Risk-A Single-Center Cross-Sectional Study. Clin Transl Gastroenterol. 2021;12(4):e00326. https://doi.org/10.14309/ctg.0000000000000326.
Margariti E, Deutsch M, Manolakopoulos S, Papatheodoridis GV. Non-alcoholic fatty liver disease may develop in individuals with normal body mass index. Ann Gastroenterol. 2012;25(1):45–51.
Ribaudo HJ, Daar ES, Tierney C, et al. Impact of UGT1A1 Gilbert variant on discontinuation of ritonavir-boosted atazanavir in AIDS Clinical Trials Group Study A5202. J Infect Dis. 2013;207(3):420–5. https://doi.org/10.1093/infdis/jis690.
Khayyat YM. Lean non-alcoholic fatty liver disease and associated metabolic disturbance: A Saudi Arabian cross-sectional study. Physiol Rep. 2021;9(14):e14949. https://doi.org/10.14814/phy2.14949.
Rana S, Bhatti AA. Association and interaction of the FTO rs1421085 with overweight/obesity in a sample of Pakistani individuals. Eat Weight Disord. 2020;25(5):1321–32. https://doi.org/10.1007/s40519-019-00765-x.
Moraes VN, Queiroz AL, Martone D, et al. Relationship between the hsa miR 150–5p and FTO gene expression in white subcutaneous adipose tissue with overweight/obesity, lipid profile and glycemia. An Acad Bras Cienc. 2020;92(4):e20200249. https://doi.org/10.1590/0001-3765202020200249.
Jalili V, Mokhtari Z, Rastgoo S, et al. The association between FTO rs9939609 polymorphism and serum lipid profile in adult women. Diabetol Metab Syndr. 2021;13(1):138. https://doi.org/10.1186/s13098-021-00754-0.
Czajkowski P, Adamska-Patruno E, Bauer W, et al. Dietary Fiber Intake May Influence the Impact of FTO Genetic Variants on Obesity Parameters and Lipid Profile-A Cohort Study of a Caucasian Population of Polish Origin. Antioxidants (Basel). 2021;10(11):1793. https://doi.org/10.3390/antiox10111793.
Acknowledgements
The research results of this article were supported by Zhangjiang Community Health Service Center of Pudong New District, Bio-X Institutes in Shanghai Jiao Tong University, and Shanghai Innovation Center of TCM Health Service of Shanghai University of Traditional Chinese Medicine. We cordially thank all the partners and staff who helped us during this cohort study, especially all the study subjects.
Funding
This study was supported in part by the Three-year action plan for Shanghai (project number: ZY (2021–2023)-0211), National Natural Science Foundation of China (81973730), Local Colleges Faculty Constitution of Shanghai MSTC 2022(22010504300), Shanghai Collaborative Innovation Center for Chronic Disease Prevention and Health Services (2021 Science and Technology 02–37), Shanghai Health Commission for Traditional Chinese Medicine Research (2022QN014), China Postdoctoral Science Foundation, No. 72 General Fund, 2022 (2022M722164).
Author information
Authors and Affiliations
Contributions
Baocheng Liu has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Baocheng Liu, Lei Zhang, and Guang He were responsible for the study concept and design. Fengwei Zhang, Ning Yu, Dong Li, and Jianying Wang were responsible for the acquisition of data. Yi Shi was responsible for the database construction. Xiangyu Zhai and Fan Yuan were responsible for experimental studies. Ruirui Wang was responsible for the literature research. Jie Li, Wu Na, and Yukun Yang were responsible for the data analysis. Jie Li and Wu Na drafted the manuscript. Jie Li, Wu Na, and Yukun Yang were responsible for the manuscript revision. All authors had a significant contribution to the intellectual content of the manuscript and approved its final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study followed the Helsinki Declaration. A standard protocol has been developed by the Shanghai Innovation Center of Traditional Chinese Medicine Health Service. This trial was approved by the Medical Ethics Committee of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine and registered in the Chinese Clinical Trial Registry (Chi CTR1800014364, registration date:2018–01-01). All study participants provided informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declared that there were no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary file 1.
Genotype and allele frequencies of polymorphisms genetic variants in NAFLD subjects.
Additional file 2:
Supplementary file 2. Genotype and allele frequencies of polymorphisms genetic variants in NAFLD subjects stratified by BMI.
Additional file 3: Supplementary file 3.
Association between clinical metabolic phenotypes with the FTO, TFAP2B, and GCKR genotype, stratified by BMI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Wu, N., Yang, Y. et al. Unique genetic variants of lean nonalcoholic fatty liver disease: a retrospective cohort study. BMC Endocr Disord 23, 11 (2023). https://doi.org/10.1186/s12902-022-01234-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01234-w