Abstract
Background
Diabetes mellitus (DM) is a global health care problem that can impose a substantial economic burden. Diabetic peripheral neuropathy (DPN) is a common microvascular complication of DM that increases the potential for morbidity and disability due to ulceration and amputation. Though there is a significant amount of variation in the primary studies on DM regarding the prevalence of DPN in Africa. Hence, this study was aimed to estimate the overall prevalence of DPN in DM patients in Africa.
Methods
PubMed, Scopus, Google Scholar, African Journals OnLine, WHO African Library, and the Cochrane Review were systematically searched online to retrieve related articles. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines was followed. Heterogeneity across the included studies was evaluated by the inconsistency index (I2). Publication bias was examined by funnel plot and Egger’s regression test. The random-effect model was fitted to estimate the pooled prevalence of diabetic peripheral neuropathy among patients in Africa. The meta-analysis was performed using the STATA™ Version 14 software.
Results
Twenty-three studies which includes 269,691 participants were included in the meta-analysis. The overall pooled prevalence of diabetic peripheral neuropathy was 46% (95% CI:36.21–55.78%). Based on the subgroup analysis, the highest prevalence of diabetic peripheral neuropathy in DM patients was reported in West Africa at 49.4% (95% CI: 32.74, 66.06).
Conclusion
This study revealed that the overall prevalence of diabetic peripheral neuropathy is relatively high in Africa. Hence, DPN needs situation-based interventions and preventive strategies, which are specific to the country. Further meta-analysis is needed to identify associated factors for the occurrence of diabetic peripheral neuropathy.
Similar content being viewed by others
Background
Diabetes melllitus (DM) is a significant health concern for many countries in the world. According to the International Diabetic Federation’s (IDF) latest estimated data, about 425 million adults in 2017 were living with diabetes globally; by 2045, this number is projected to rise to 629 million. In Africa, by 2017, 39 million people were living with diabetes and by 2045, this number is projected to rise to 82 million [1]. Diabetes is also a significant cause of death around the world, with estimates being that in 2015 diabetes directly caused 1.6 million deaths worldwide [2]. Additionally, over the past decade, the prevalence of diabetes has risen faster in low and middle-income countries than in high-income countries [3].
Morbidity and mortality in patients with DM is mainly attributed to microvascular and macrovascular complications [4]. Diabetic peripheral neuropathy (DPN) is a common microvascular complication of DM that increases the potential for morbidity and disability due to ulceration and amputation [5]. DPN is an asymmetrical, sensorimotor polyneuropathy that is caused by metabolic and microvascular changes that result from long-term hyperglycaemia and metabolic disorder [6]. Moreover, DPN in its earliest stages leads to segmental demyelination, which subsequently results in delayed nerve conduction velocity [7].
The prevalence of DPN varies widely in the literature. This is due to differences in the diagnostic criteria employed, types of diabetes, the different methods of patient selection, and the sample size [8, 9]. However, it has been estimated that the prevalence of DPN is 8.4% in China [10], 48.1% in Sri Lanka [11], 29.2% in India [12], 56.2% in Yemen [9], 39.5% in Jordan [13], 71.1% in Nigeria [14], 16.6% in Ghana [15], and 29.5% in Ethiopia [16].
Peripheral nerve damage in diabetic patients is mostly irreversible. This has led health care professionals to focus on prevention as well as the identification of modifiable risk factors [17]. Studies suggest that numerous risk factors are responsible for DPN in DM patients including age, gender, duration of diabetes, the presence of microvascular complications, hypertension, area of residence, body mass index, glycated haemoglobin (HbA1c) level, alcohol intake, hyperglycaemia, cigarette smoking, physical inactivity, and marital status [12, 18,19,20,21,22,23,24,25].
Patients with DPN often suffer from the loss or absence of a protective sensation in the lower extremities leading to balance problems [26], risk of foot ulcerations [22], pain and disrupted sleep patterns [27], cardiovascular morbidity and mortality [19], reduced quality of life [28], and increased cost of treatment [29]. Previous studies have indicated that for those with high-risk diabetic neuropathy, proper management and early screening can minimize the occurrence of ulcers by 60% and amputations by 85% [30]. Moreover, different primary studies in Africa show the magnitude of DPN as a health issue in the region. However, these studies have demonstrated substantial variation regarding its prevalence. Therefore, this study was aimed to estimate the pooled prevalence of DPN in patients with DM in Africa. Findings from the current study would serve as a benchmark for policymakers to implement appropriate preventative measures and to alleviate the pressing problem of DPN.
Methods
Search strategy and database
To extract all relevant literature, electronic databases such as PubMed, Google Scholar, African Journals of OnLine, Scopus, Web of Science, WHO African Library, and the Cochrane Review were searched. In addition, a hand search of grey literature and other related articles were conducted to retrieve additional relevant articles. All electronic sources of information were searched for the period of January 1st, 2000 to August 22nd, 2019. The search was deployed using the following MeSH and free-text terms: “peripheral neuropathy”, “diabetic neuropathy”, “diabetic polyneuropathy”, “diabetes mellitus”, and “Africa”. Boolean operators like “AND” and “OR” were used to combine search terms.
Eligibility criteria
Studies were included if they met the following criteria: (1) studies reported their outcome variable as prevalence of DPN, (2) articles were published in peer-reviewed journals or grey literature, (3) articles were published in English between 2000 to 2019, and (4) studies were conducted using an African population. Studies were excluded on any one of the following conditions: (1) the article was not fully accessible (i.e., the full text was not available) at the time of our search, (2) it was a duplicate citation, (3) the article had a sub-standard quality score per stated criteria, (4) the study was not relevant to DPN, (5) the study involved peripheral neuropathy not related to DM, and (6) the patients in the study had comorbidities of human immunodeficiency virus (HIV), tuberculosis, and/or chemotherapy.
Selection and quality assessment
Data were extracted using a pre-piloted data extraction format prepared in Microsoft™ Excel. The extracted information from the literature included author names, year of publication, study area, study design, sample size, data collection year, data collection method, reported prevalence, and its 95% confidence interval. The data were extracted by three independent authors. The methodological and overall quality of each article was assessed by both authors based on the modified version of the Newcastle-Ottawa Scale (NOS) for cross-sectional studies [31]. Studies which scored ≥5 out of 10 points in three domains of the modified NOS for a cross-sectional study were included in the analysis [32]. Any disagreements at the time of data abstraction were reconciled by discussion and consensus, (Supplementary file 1).
Statistical analysis
To estimate the pooled prevalence of DPN, a meta-analysis using the random effect DerSimonian and Laird model was deployed. Cochran’s Q chi-square statistics and I2 statistical test were conducted to assess the random variations between primary studies [33]. To minimize the random variations between the point estimates of the primary study, meta-regression, subgroup analyses, and sensitivity analysis were performed to investigate the sources of heterogeneity. Publication bias was assessed by visual inspection of a funnel plot. In addition, an Egger test was conducted and a p ≤ 0.05 was considered statistically significant for the presence of publication bias [34, 35]. The meta-analysis was performed using the STATA™ version 14 statistical software for Windows™.
Data synthesis and reporting
To estimate the pooled prevalence of DPN in patients with DM, this systematic review and meta-analysis was carried out using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [36], and PRISMA checklist has been used. The weighted prevalence of DPN in patients with DM was presented using a forest plot.
Results
Search results
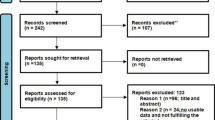
In total, 1278 studies were retrieved, of which, 1261 were found from six international databases and the remaining 17 were found through manual searches. The articles retrieved from the databases were as follows: PubMed (161), Scopus (53), Google Scholar (507), WHO African Library (3), Cochrane Reviews (7), and the African Journals OnLine (530). Of these papers, 659 duplicate records were identified and removed. From the remaining 619 articles, 492 articles were excluded after reading of titles and abstracts based on the pre-defined eligibility criteria. Finally, 127 full-text articles were read and assessed. After applying the pre-defined criteria and quality assessment, 23 articles were eligible for review and included in the final analysis (Fig. 1).
Baseline characteristics of the included studies
A total of 23 studies with 269,691 study participants were included in this meta-analysis. Overall information regarding the prevalence of DPN in DM patients was obtained from various countries across Africa including 10 studies from Nigeria [14, 18,19,20, 22, 37,38,39,40,41], four from Ethiopia [16, 24, 42, 43], two from Cameroon [23, 44], two from Sudan [45, 46], two from Egypt [47, 48], and one each from Ghana [15], Uganda [49], and Tanzania [50]. The highest and lowest prevalence of DPN was 83.4% [22] and 7.5% [41] respectively, which were reported from Nigeria. Study sample sizes ranged from 50 to 524 participants. Moreover, based on the modified Newcastle-Ottawa quality score assessment, all 23 articles fulfilled the required quality score (Table 1).
Prevalence of DPN
The result of this meta-analysis using a random effects model showed that the pooled prevalence of DPN was 46% (95% CI: 36.21–55.78) (Fig. 2) with significant heterogeneity being observed (I2 = 98.7%; p ≤ 0.001).
Subgroup analysis
The presence of significant heterogeneity among the primary studies required that we conduct subgroup analysis. As a result, in order to ascertain the sources of heterogeneity we deployed subgroup analysis using study area (geographic indicator) as the variable of interest. From this we found that the highest prevalence of DPN was observed in a study conducted in West Africa 49.4% (95% CI: 32.74, 66.06) (Fig. 3).
Meta-regression analysis
To investigate the possible source(s) of variation across the included studies, we performed meta-regression analysis using publication year and sample size as covariates of interest. However, the result of this meta-regression analysis showed that both covariates were not significantly associated with the presence of heterogeneity, (Table 2).
Publication bias
To identify the presence of publication bias, both a funnel plot and Egger’s test were performed. Visual inspection of the funnel plot showed an asymmetrical distribution, which indicated the presence of publication bias (Fig. 4). The finding of publication bias was affirmed following the Egger’s test (p = 0.024).
Sensitivity analysis
We conducted a sensitivity analysis to assess the effect of any individual study on the pooled effect size. Our analyses using a random effects model revealed that no single study affected the overall prevalence of DPN (Fig. 5).
Discussion
In this systematic review and meta-analysis, the overall prevalence of DPN was 46% (95%CI: 36.21, 55.78). This finding was in line with a systematic review and meta-analysis conducted in Iran which yielded a prevalence of 53% [52]. In contrast, the prevalence found in our study was higher than a systematic review and meta-analysis conducted in developed countries, which reported a prevalence of 35.78% [53]. This variation could be the result of different diagnostic criteria for diabetic neuropathy, and early diagnosis and treatment in developed countries.
DPN prevalence varied greatly in the included studies, ranging from 7.5% [41] to 83.4% [22]. However, our subgroup analysis based on study area showed that the highest pooled prevalence of DPN was observed from studies done in West Africa (49.4%; 95% CI: 32.74, 66.06) and the lowest was observed in Central Africa (35.9%; 95% CI: 29.51, 42.32). This discrepancy could be explained by studies using different diagnostic criteria for diabetic neuropathy, the quality of the health care service, and the duration and severity of diabetes. The findings of this meta-analysis have implications for clinical practice. Specifically, estimating the pooled prevalence of DPN will indicate where preventative strategies are needed most and may reflect the quality of healthcare given to patients in a particular area.
This systematic review and meta-analysis was conducted based on PRISMA guidelines for literature reviews. In addition, publication bias was quantified using Egger’s regression statistical test, and NOS was used to assess the quality of the included studies. To the best of our knowledge this is the first study on the prevalence of DPN in DM patients from Africa which may be helpful for future researchers, public health practitioners, and health care policymakers.
This study was conducted with the use of a comprehensive search strategy to incorporate the studies involving African patients. All of the included studies were observational studies with high methodological quality based on NOS assessment. In addition, the inclusion of previously published studies that met our inclusion criteria further strengthened our meta-analysis. There are several limitations to this review which must be acknowledged and may inform future research. First, we only used English language articles although our target was the African content which could be in several other languages such as Spanish, French, or Portuguese. Also, our study was exclusively driven by hospital-based data, which reduced the community-based capture on this topic. Finally, we did not explore the predictors of DPN in DM patients.
Conclusion
This study revealed that the overall prevalence of DPN was relatively high in Africa. Hence, African nations need to implement situation-based interventions and preventive strategies in order to try to curb this debilitating disease. In addition, policymakers and other concerned bodies need to give special attention to improve healthcare delivery for patients with DM to reduce the risk of DPN. Furthermore, further research is needed to identify associated factors for the development of DPN in patients with DM.
Availability of data and materials
The data analyzed during the current meta-analysis is available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- DM:
-
DIABETES mellitus
- DPN:
-
Diabetic peripheral neuropathy
- NOS:
-
Newcastle-Ottawa Scale
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
References
International Diabetic Federation. Diabetes Atlas. 8th ed; 2017.
World Health Organization. Guidelines on second-and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus: World Health Organization; 2018. https://apps.who.int/iris/handle/10665/272433. Accessed 10 Sept 2019.
Roglic G. WHO global report on diabetes: a summary. Int J Noncommun Dis. 2016;1(1):3.
Kumar KH, Kota S, Basile A, Modi K. Profile of microvascular disease in type 2 diabetes in a tertiary health care hospital in India. Ann Med Health Sci Res. 2012;2(2):103–8.
Genuth S, Eastman R, Kahn R, Klein R, Lachin J, Lebovitz H, Nathan D, Vinicor F. Implications of the United Kingdom prospective diabetes study. Diabetes Care. 2003;26:S28.
Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik R, Spallone V, Vinik A. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments (Diabetes Care (2010) 33,(2285-2293)). Diabetes Care. 2010;33(12):2725.
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(1):S4.
Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120(1):1–34.
Al Washali A, Azuhairi A, Hejar A, Amani Y. Prevalence and associated risk factors of diabetic peripheral neuropathy among diabetic patients in national center of diabetes in Yemen. Int J Public Health Clin Sci. 2014;1(1):141–50.
Lu B, Hu J, Wen J, Zhang Z, Zhou L, Li Y, Hu R. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes–ShangHai diabetic neuRopathy epidemiology and molecular genetics study (SH-DREAMS). PLoS One. 2013;8(4):e61053.
Katulanda P, Ranasinghe P, Jayawardena R, Constantine GR, Sheriff MR, Matthews DR. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetol Metab Syndr. 2012;4(1):21.
Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of development of peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes invest. 2014;5(6):714–21.
Khawaja N, Abu-Shennar J, Saleh M, Dahbour SS, Khader YS, Ajlouni KM. The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol Metab Syndr. 2018;10(1):8.
Owolabi MO, Ipadeola A. Total vascular risk as a strong correlate of severity of diabetic peripheral neuropathy in Nigerian Africans. Ethn Dis. 2012;22(1):106–12.
Yeboah K, Agyekum JA, Owusu Mensah RN, Affrim PK, Adu-Gyamfi L, Doughan RO, Adjei AB. Arterial stiffness is associated with peripheral sensory neuropathy in diabetes patients in Ghana. J Diabetes Res. 2018;2018:2320737.
Worku D, Hamza L, Woldemichael K. Patterns of diabetic complications at jimma university specialized hospital, southwest ethiopia. Ethiop J Health Sci. 2010;20(1):33–9.
Won JC, Park TS. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab. 2016;31(2):230–8.
Olamoyegun M, Ibraheem W, Iwuala S, Audu M, Kolawole B. Burden and pattern of micro vascular complications in type 2 diabetes in a tertiary health institution in Nigeria. Afr Health Sci. 2015;15(4):1136–41.
Bello A, Biliaminu S, Wahab K, Sanya E. Distal symmetrical polyneuropathy and cardiovascular autonomic neuropathy among diabetic patients in Ilorin: prevalence and predictors. Niger Postgrad Medl J. 2019;26(2):123.
Oguejiofor O, Odenigbo C, Oguejiofor C. Evaluation of the effect of duration of diabetes mellitus on peripheral neuropathy using the United Kingdom screening test scoring system, bio-thesiometry and aesthesiometry. Niger J Clin Pract. 2010;13(3):240–7.
Azura M, Adibah H, Juwita S. Risk factor of peripheral neuropathy among newly diagnosed type 2 diabetic patients in primary care clinic. Int J Collaborative Res Int Med Public Health. 2012;4(11):1858.
Ede O, Eyichukwu GO, Madu KA, Ogbonnaya IS, Okoro KA, Basil-Nwachuku C, Nwokocha KA. Evaluation of peripheral neuropathy in diabetic adults with and without foot ulcers in an African population. J Biosci Med. 2018;6(12):71–8.
Tamba SM, Ewane ME, Bonny A, Muisi CN, Nana E, Ellong A, Mvogo CE, Mandengue SH. Micro and macrovascular complications of diabetes mellitus in Cameroon: risk factors and effect of diabetic check-up-a monocentric observational study. Pan Afr Med J. 2013;15(1):3–5.
Jember G, Melsew YA, Fisseha B, Sany K, Gelaw AY, Janakiraman B. Peripheral sensory neuropathy and associated factors among adult diabetes mellitus patients in Bahr Dar, Ethiopia. J Diabetes Metab Disord. 2017;16(1):16.
Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS One. 2019;14(2):e0212574.
Herrera-Rangel A, Aranda-Moreno C, Mantilla-Ochoa T, Zainos-Saucedo L, Jáuregui-Renaud K. The influence of peripheral neuropathy, gender, and obesity on the postural stability of patients with type 2 diabetes mellitus. J Diabetes Res. 2014;2014:787202.
Hoffman DL, Sadosky A, Alvir J. Cross-national burden of painful diabetic peripheral neuropathy in Asia, Latin America, and the Middle East. Pain Practice. 2009;9(1):35–42.
Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, Engelgau MM, Kaplan RM, Herman WH. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25(12):2238–43.
Mehra M, Merchant S, Gupta S, Potluri RC. Diabetic peripheral neuropathy: resource utilization and burden of illness. J Med Econ. 2014;17(9):637–45.
G. N. Progress in the diagnosis of diabetic peripheral neuropathy. Chin J Pract Intern Med. 2007;7:487–9.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Shiferaw WS, Akalu TY, Aynalem YA. Prevalence of erectile dysfunction in patients with diabetes mellitus and its association with body mass index and Glycated hemoglobin in Africa: a systematic review and meta-analysis. Int J Endocrinol. 2020;2020:2–3.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. American multinational publishing company: Wiley; 2011.
Egger M, Davey-Smith G, Altman D. Systematic reviews in health care: meta-analysis in context. American multinational publishing company: Wiley; 2008.
Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Stat Med. 2008;27(5):746–63.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Ugoya SO, Echejoh GO, Ugoya TA, Agaba EI, Puepet FH, Ogunniyi A. Clinically diagnosed diabetic neuropathy: frequency, types and severity. J Natl Med Assoc. 2006;98(11):1763.
Adeniyi AF, Aiyegbusi OS, Ogwumike OO, Adejumo PO, Fasanmade AA. Habitual physical activity, peripheral neuropathy, foot deformities and lower limb function: characterising prevalence and interlinks in patients with type 2 diabetes mellitus. J Endocrinol Metab Diabetes S Afr. 2015;20(2):101–7.
Ojieabu WA, Odusan O, Ojieabu NI, Oku LM. Evaluation of prevalence of micro-and macrovascular complications among elderly type 2 diabetes patients in a health facility. Afr J Biomed Res. 2017;20(2):131–5.
Ogbera AO, Adeleye O, Solagberu B, Azenabor A. Screening for peripheral neuropathy and peripheral arterial disease in persons with diabetes mellitus in a Nigerian University teaching hospital. BMC Res Notes. 2015;8(1):533.
Mba I. Diabetes mellitus in Abia State University teaching hospital aba, a 30 months retrospective study. J Med Investig Pract. 2001;2(1):48–51.
Gill G, Gebrekidan A, English P, Wile D, Tesfaye S. Diabetic complications and glycaemic control in remote North Africa. QJM. 2008;101(10):793–8.
Jarso G, Ahmed A, Feleke Y. The prevalence, clinical features and management of periphral neuropathy among diabetic patients in Tikur Anbessa and St. Paul's Specialized University hospitals, Addis Ababa, Ethiopia. Ethiop Med J. 2011;49(4):299–311.
Kuate-Tegueu C, Temfack E, Ngankou S, Doumbe J, Djientcheu V, Kengne A. Prevalence and determinants of diabetic polyneuropathy in a sub-Saharan African referral hospital. J Neurol Sci. 2015;355(1–2):108–12.
Awadalla H, Noor SK, Elmadhoun WM, Almobarak AO, Elmak NE, Abdelaziz SI, Sulaiman AA, Ahmed MH. Diabetes complications in Sudanese individuals with type 2 diabetes: overlooked problems in sub-Saharan Africa? Diabetol Metab Syndr. 2017;11:S1047–51.
Mohmad AH, Hassan A. Correlation between retinopathy, nephropathy and peripheral neuropathy among adult Sudanese diabetic patients. Sudan J Me Sci. 2011;6(1):28–9.
Khalil SA, Megallaa MH, Rohoma KH, Guindy MA, Zaki A, Hassanein M, Malaty AH, Ismael HM, Kharboush IF, Kafash E. Prevalence of chronic diabetic complications in newly diagnosed versus known type 2 diabetic subjects in a sample of Alexandria population, Egypt. Curr Diabetes Rev. 2019;15(1):74–83.
Mohamed MM, Ali RA, Hamdoon MN. Frequency and determinants of peripheral neuropathy in diabetic children in Sohag, Egypt. J Behav Brain Sci. 2019;9(4):184–94.
Kisozi T, Mutebi E, Kisekka M, Lhatoo S, Sajatovic M, Kaddumukasa M, Nakwagala FN, Katabira E. Prevalence, severity and factors associated with peripheral neuropathy among newly diagnosed diabetic patients attending Mulago hospital: a cross-sectional study. Afr Health Sci. 2017;17(2):463–73.
Amour AA, Chamba N, Kayandabila J, Lyaruu IA, Marieke D, Shao ER, Howlett W. Prevalence, patterns, and factors associated with peripheral neuropathies among diabetic patients at tertiary Hospital in the Kilimanjaro Region: descriptive cross-sectional study from north-eastern Tanzania. Int J Endocrinol. 2019;2019:4–6.
Oguejiofor OC, Onwukwe CH, Ezeude CM, Okonkwo EK, Nwalozie JC, Odenigbo CU, Oguejiofor CB. Peripheral neuropathy and its clinical correlates in type 2 diabetic subjects without neuropathic symptoms in Nnewi, South-Eastern Nigeria. J Diabetol. 2019;10(1):21.
Sobhani S, Asayesh H, Sharifi F, Djalalinia S, Baradaran HR, Arzaghi SM, Mansourian M, Rezapoor A, Ansari H, Masoud MP. Prevalence of diabetic peripheral neuropathy in Iran: a systematic review and meta-analysis. J Diabetes Metab Disord. 2014;13(1):97.
de Souza LR, Debiasi D, Ceretta LB, Simões PW, Tuon L. Meta-analysis and meta-regression of the prevalence of diabetic peripheral neuropathy among patients with type 2 diabetes mellitus. Int Arch Med. 2016;9:6–9.
Acknowledgements
We would like to thanks Dr. Ryan Bell for detail English language edition and valuable feedback on the entire document.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
WSS and TYA developed the protocol and were involved in the design, study selection, data extraction, statistical analyses, and developing the initial drafts of the manuscript. YAA, YW, and TYA were involved in data extraction, quality assessment, statistical analyses, and revising the manuscript. WSS and YAA prepared the final draft of the manuscript. All authors read and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Supplementary file 1.
Methodological quality assessment of cross-sectional studies using the modified Newcastle-Ottawa Scale (NOS).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shiferaw, W.S., Akalu, T.Y., Work, Y. et al. Prevalence of diabetic peripheral neuropathy in Africa: a systematic review and meta-analysis. BMC Endocr Disord 20, 49 (2020). https://doi.org/10.1186/s12902-020-0534-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-020-0534-5