Abstract
Background
Idiopathic scrotal calcinosis (ISC) is a manifestation of idiopathic calcinosis cutis, and its etiology is still unknown.
Case presentation
We report a 36-year-old patient manifested multiple gradually increasing yellowish-white scrotal nodules with occasional itching and stinging in the past 6 years and was successfully cured via surgical excision. The laboratory test combined with pathological analysis confirmed the diagnosis of ISC. Like pathological calcinosis in other soft tissues, a large amount of collagen fiber deposition was observed around the calcification nodule, suggesting that abnormal collagen fiber deposition might be an important factor leading to idiopathic calcinosis in the scrotum. Moreover, koilocytes, which indicate human papillomavirus (HPV) infection, were also detected around calcified nodules, indicating the potential pathogenic role of HPV infection in ISC.
Conclusions
Here, we report that ISC shows abnormal excessive deposition of collagen fibers around calcified nodules, which may be a vital factor contributing to the disease. Furthermore, combined with the literature review, a new pathogenic mechanism of ISC is proposed, and the site specificity of scrotal calcinosis is explained, providing a basis for further exploration of the pathogenic mechanism of ISC.
Similar content being viewed by others
Background
Calcinosis cutis is a variety of skin calcium deposition diseases caused by the deposition of insoluble calcium salt crystals in human skin, manifesting as multiple local nodules in the skin [1]. According to its etiology, it can be divided into 5 subtypes: dystrophic calcification, metastatic calcification, idiopathic calcification, tumoral calcification, and calciphylaxis [2, 3]. Idiopathic scrotal calcinosis (ISC), which is an idiopathic calcinosis cutis, is characterized by multiple symptomless yellowish-white nodules of varying sizes that occur particularly in the subcutaneous tissue of the scrotum of 20–40 years old males [4]. Although some studies have speculated that this may be caused by the abnormal calcification of epidermoid cysts, the contents of dilated exocrine ducts, or HPV infection, it remains unclear why and how calcinosis occurs specifically in the male scrotum [4,5,6,7]. In this case, our observations, especially the deposition of a large number of collagen fibers around the lesions, are described in detail, and by integrating the case and known relevant studies, we propose a new mechanism model of calcification in ISC, which might advance our understanding of ISC.
Case presentation
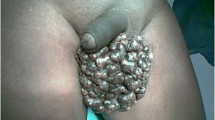
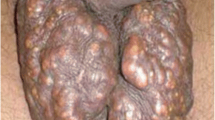
Here, we report the case of a 36-year-old man who was admitted to the Department of Urology with complaints of multiple gradually increasing scrotal nodules with occasional itching and stinging in the past 6 years. These nodules were yellowish-white and firm, while the surfaces of some of the nodules were broken and exhibit chalky discharge (Fig. 1A). The abnormal appearance of these nodules strongly affected the patient’s sexual life and self-esteem. The patient denied any history of infection, trauma or inflammatory disease of the scrotum, including orchitis, epididymitis and others, and stated that he had never had parathyroid or metabolic diseases and no family history of similar diseases. Further laboratory tests revealed routine blood and urine tests, and the phosphorus, calcium, and uric acid levels were within the normal ranges. His blood parathyroid hormone level was also comparable to normal. Ultrasound of the scrotum showed multiple, varying-sized and hypoechoic nodules in the subcutaneous tissue of the scrotum, some of which were fused into a mass, and multiple punctate hyperechoic areas were observed within the hypoechoic areas, followed by a faint sound shadow (Fig. 1B). The sizes of the bilateral testicles, right epididymis and left epididymis were normal. Preoperative routine examinations including chest X-ray and electrocardiogram did not reveal any abnormalities. Then, the patient underwent general anaesthesia for complete scrotal lesion resection. One week after the operation, the incision had healed well (Supplemental Fig. 1), and there was no abnormality or recurrence at the nine-month postoperative follow-up, which is still continuing (Fig. 1C). The self-esteem and quality of sexual life of the patient significantly improved after the surgery.
The clinical manifestations of the ISC patient. (A) A general photograph of the preoperative scrotum. The surface of the scrotum exhibited multiple, variable-sized and hard nodules with smooth, well-defined borders that partially fused into the mass, whereas the surfaces of some of the nodules were broken and released chalky discharge. (B) Ultrasound image of the scrotum. Several hypoechoic areas of different sizes could be detected in the subcutaneous region of the scrotum, while multiple punctate hyperechoic areas could be observed within the hypoechoic areas, followed by a faint sound shadow. The white asterisks represent these nodules. (C) A general photograph of the scrotum 9 months after the operation
The lesions were further examined by pathology. Histopathological examination of the representative nodules revealed chronic inflammatory cell infiltration, massive calcification and multinucleated giant cell reactions around the nodules, all of which are in accordance with idiopathic scrotal calcinosis (Fig. 2A-B). Besides, we also observed large numbers of koilocytes around the nodules, as shown in Fig. 2C, indicating that the potential HPV infection of ISC and HPV infection may contribute to the pathogenesis of ISC [5]. The HE staining results also showed abundant fibers which were stained red by Eosin dyes around the calcified nodules (Fig. 2D). Masson staining was further used to detect the type of fibers around the lesions and the results testified the abundant fibrous tissues were collagen fibers (Fig. 3). The large number of collagens deposited around the lesions, suggested that excessive collagen might be a vital pathogenic factor in ISC.
HE staining of the excised scrotum tissues. (A) Multiple and varying-sized calcified nodules are observed in the excised lesion of the scrotum. The dashed boxes are enlarged areas, and the black asterisks represent calcified nodules. The black arrowheads indicated the red stained fibrous tissue. (B) Enlarged area of dotted box 1 shown on the left. The foreign body reacting to giant cell (FBGC) labelled with a black arrow is detected around the calcified nodules. (C) The enlarged area of dotted box 2 shown on the left. Koilocytes, which are characteristic of HPV infection, are labelled with black arrows surrounding calcified nodules. (D) The enlarged area of dotted box 3 shown on the left. The fibrous tissue which was stained red surrounding calcified nodules by the Eosin dyes are labelled with black arrowheads
Schematic representation of the proposed pathogenic mechanism of ISC. HPV infection and other microinjury are the initiated harmful stimuli that cause microinflammation in the scrotum. The highly abundant elastic fibers and smooth muscle cells (SMCs) in the scrotum provide a unique microenvironment for microcalcification formation through the release of elastin-derived peptides (EDPs) or transdifferentiation into osteoblast-like cells. The high expression of collagen fibers in the scrotum provides a vital scaffold and further promotes the aggregation of calcification
Discussion
Physiological calcification is a process in which ionized calcium (Ca2+) and PO43− are deposited in bones and teeth under a precise regulatory mechanism, whereas pathological calcification is the ectopic deposition of calcium salts in the skin, blood vessels, joints, tumors, etc [3]. Collagen fibers, the major components of the extracellular matrix, have been shown to not only function as scaffolds for calcium deposition but also play important roles in vesicle-mediated microcalcification nucleation and distribution during calcification [8]. It has been reported that increased expression of collagen I can facilitate the formation of vascular calcification [9]. In the process of atherosclerotic plaque formation, there is an intense fibrotic response and a large, disordered accumulation of collagen before calcification begins [10]. In this case, we observed excessive collagen deposition around the calcified nodules. The similar appearance of highly expressed collagen fibers in this ectopic calcinosis strongly suggests that collagen likely promotes calcification in ISCs. Furthermore, the deposition of collagen fibers can also be a downstream manifestation of the foreign body reaction. To further clarify the role of collagen fibers in ISC, it is reasonable to utilize relevant inhibitors that target the inflammatory response of foreign body reaction or collagen fiber deposition to detect the effect on downstream calcinosis in the ISC model.
Scrotal calcinosis was first described by Lewinski in 1883 but was first reported in 1970 by Shapiro et al. [11, 12]. However, the reason for site-specific scrotal calcinosis in ISCs is unclear. It is known that in the process of calcification of vascular media, the transdifferentiation of smooth muscle cells into osteoblast-like cells and the further secretion of microvesicles from these cells are crucial in the process of microcalcification nucleation [3, 8, 13]. In addition, the proteolytic degradation products of elastic fibers, elastin-derived peptides (EDPs), play a key role in the calcification of atherosclerosis by providing micronucleation sites. EDPs, as potent osteogenic factors, can upregulate the expression of key downstream osteogenic factors, such as α-SMA and Runx2, thereby promoting the osteogenic transdifferentiation of multiple cell types, such as mesenchymal stromal cells (MSCs), vascular smooth muscle cells (VSMCs), and myofibroblasts [14, 15]. Interestingly, compared with other body skin, highly abundant elastic fibers and smooth muscles are distinctive features of the scrotum, providing unique conditions and microenvironments for calcification, which could explain the site specificity of calcinosis in the scrotum. Moreover, ISC has been reported to be associated with HPV infection, and koilocytes, which indicate HPV infection around calcified nodules, were detected in this patient, further suggesting that microinflammation caused by HPV infection may be the driving force of calcinosis. Further testing of the causal relationship between HPV and ISC in clinical practice and animal models is promising.
Currently, the most effective treatment for ISC is still complete surgical excision, but surgical resection also has the risk of large skin defects, wound infection, postoperative residual disease or lesion recurrence. On the basis of the pathogenesis of ISC proposed in this paper, multiple aspects of ISC intervention might be effective. First, patients infected with HPV can receive active anti-HPV treatment to prevent local microinflammation in the scrotum. In addition, some drugs that have been proven to be effective in treating vascular calcification may also be effective for treating ISC, such as pyridoxine, SO2, and verapamil, which interfere with collagens and EDPs [16,17,18].
Conclusion
Here, we reported that there was excessive abnormal deposition of collagen fibers around calcified nodules in ISC, which might be the key factor causing this disease. Combined with the literature review, a new pathogenic mechanism of ISC was proposed (Fig. 4), and the site specificity of calcinosis of the scrotum was explained, which provides a theoretical basis for further exploration of the pathogenic mechanism of ISC.
Data availability
All data involved in this study are presented in this manuscript and supplemental material.
References
Le C, Bedocs PM. Calcinosis Cutis. In: StatPearls. edn. Treasure Island (FL) companies. Disclosure: Paul Bedocs declares no relevant financial relationships with ineligible companies.; 2024.
Elahmar H, Feldman BM, Johnson SR. Management of Calcinosis Cutis in Rheumatic diseases. J Rheumatol. 2022;49(9):980–9.
Proudfoot D. Calcium Signaling and tissue calcification. Cold Spring Harb Perspect Biol 2019, 11(10).
Ye D, Ma X, Yang X. Scrotal calcinosis: a case report and literature review. Am J Clin Exp Urol. 2022;10(3):194–8.
Lu C, Hong H, Lu C, Ko Y. Human papillomavirus-70 infection as a possible pathogenesis of eruptive scrotal calcinosis. Dermatol Sin. 2017;35(3):152–4.
Ito A, Sakamoto F, Ito M. Dystrophic scrotal calcinosis originating from benign eccrine epithelial cysts. Br J Dermatol. 2001;144(1):146–50.
Feng L, Shulin G, Jinhua W, Zhongxiang L, Peiyan L, Yanhua W, Jiangping X. Idiopathic calcinosis of scrotum: a case report and review of the literature. Heliyon. 2022;8(9):e10762.
Zhao Y, Sun Z, Li L, Yuan W, Wang Z. Role of collagen in vascular calcification. J Cardiovasc Pharmacol. 2022;80(6):769–78.
Zeng Y, Wu J, He X, Li L, Liu X, Liu X. Mechanical microenvironment regulation of age-related diseases involving degeneration of human skeletal and cardiovascular systems. Prog Biophys Mol Biol. 2019;148:54–9.
Blaser MC, Aikawa E. Roles and regulation of Extracellular vesicles in Cardiovascular Mineral metabolism. Front Cardiovasc Med. 2018;5:187.
Dr.Lewinski. Lymphangiome der Haut mit verkalktem Inhalt 1883, Vol.91(No.2):371–373.
Shapiro L, Platt N, Torres-Rodriguez VM. Idiopathic calcinosis of the scrotum. Arch Dermatol. 1970;102(2):199–204.
Burgess KA, Herrick AL, Watson REB. Systemic sclerosis skin is a primed microenvironment for soft tissue calcification-a hypothesis. Rheumatology (Oxford). 2021;60(6):2517–27.
Andrault PM, Panwar P, Mackenzie NCW, Bromme D. Elastolytic activity of cysteine cathepsins K, S, and V promotes vascular calcification. Sci Rep. 2019;9(1):9682.
Heinz A. Elastases and elastokines: elastin degradation and its significance in health and disease. Crit Rev Biochem Mol Biol. 2020;55(3):252–73.
Chang KC, Liang JT, Tsai PS, Wu MS, Hsu KL. Prevention of arterial stiffening by pyridoxamine in diabetes is associated with inhibition of the pathogenic glycation on aortic collagen. Br J Pharmacol. 2009;157(8):1419–26.
Cai H, Wang X. Effect of sulfur dioxide on vascular biology. Histol Histopathol. 2021;36(5):505–14.
Chen NX, Kircelli F, O’Neill KD, Chen X, Moe SM. Verapamil inhibits calcification and matrix vesicle activity of bovine vascular smooth muscle cells. Kidney Int. 2010;77(5):436–42.
Acknowledgements
We thank the patient in this study. We also thank all the medical teams, including those in the departments of ultrasonic diagnosis, pathology and anaesthesia surgery, for their kind assistance in surgery and treatment.
Funding
None.
Author information
Authors and Affiliations
Contributions
Dr. Qisheng Tang and Dr. Ning Ning designed the study and revised the manuscript. Dr. Bo Liu and Gongquan Xu performed the histological staining and wrote the manuscript. Dr. Hao Li and Dr. Guocheng Lu participated in the treatment and follow-up of the patient.
Corresponding authors
Ethics declarations
This research was approved by the Ethics Committee of Tangdu Hospital of Fourth Military Medical University (Clinical Trial Number: 202109-25).
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplementary Fig. 1
: A general photograph of the well-healed scrotum at 1 week after the operation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, B., Xu, G., Li, H. et al. Excessive collagen fiber deposition in idiopathic scrotal calcinosis: a case report. BMC Urol 24, 212 (2024). https://doi.org/10.1186/s12894-024-01601-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-024-01601-w