Abstract
Background
Xanthogranulomatous pyelonephritis (XGP) is a rare chronic pyelonephritis that often mimics other renal diseases, when combined with autosomal dominant polycystic kidney disease(ADPKD), preoperative diagnosis is exceedingly difficult. It is important for clinicians to be aware of an XGP with ADPKD since a misdiagnosis can lead to unnecessary surgical intervention.
Case presentation
Here, we report a case of a 66-year-old female with a history of bilateral ADPKD and urinary tract infection admitted to our hospital due to right flank pain, feeble, and low-grade fever. Contrast-enhanced ultrasound revealed a malignant mass of the right kidney suspected to be a cystic renal cell carcinoma with polycystic kidney disease. In addition, contrast-enhanced computed tomography (CT) and fluorine 18 fluorodeoxyglucose PET/CT (18F FDG PET/CT) showed similar results. Subsequently, the patient underwent a right radical nephrectomy, but histopathological examination revealed XGP with ADPKD. On the follow-up, the patient's symptoms were relieved.
Conclusions
XGP should be kept in mind during the differential diagnosis of renal masses with ADPKD even in the absence of characteristic clinical symptoms and imaging manifestations.
Similar content being viewed by others
Background
Xanthogranulomatous pyelonephritis (XGP) is a severe chronic infectious renal disease, which was first described as lipid-filled macrophages in a granulomatous inflammatory process in 1916 by Schlagenhaufer [1]. XGP can occur at any age, but most often occur in immunocompromised middle-aged women [2, 3]. The exact etiology of XGP remains unclear, and often develops secondary to recurrent urinary tract infections and nephrolithiasis obstructions [4]. This is rare case reporting XGP with ADPKD.
Case presentation
A 66-year-old female with a history of bilateral ADPKD and urinary tract infection was admitted due to pain in the right flank. The pain in the right flank started approximately 15 days ago, remained persistent, and presented with a low-grade fever and weakness. Although her body temperature was approximately 36.7 °C, she complained of a low basal body temperature throughout the day. There is a family history of ADPKD, but she denied any associated hematuria, dysuria, or weight loss. Physical examination showed no bilateral kidney area bulge or percussed pain. Urinalysis revealed normal red blood cell levels, with white blood cell levels significantly increased to 105.45/μL (normal, 0–28/μL), and the blood tests showed white blood cell levels of 12.8 × 109/L (normal, 3.5 × 109–9.5 × 109/L), red blood cell levels of 3.05 × 1012/L (normal, 3.8 × 1012–5.1 × 1012/L), and elevated C-reactive protein levels (167 mg/L). Renal function tests showed a serum creatinine level of 145 μmol/L (normal, 41-81 μmol/L) and BUN levels, total carbon dioxide, uric acid, and glomerular filtration rate all within normal limits. Urine culture suggested an Escherichia coli (E. coli) infection. Blood culture was not performed. Before admission, the patient underwent conventional ultrasound imaging to determine her symptoms, etiology and assess the right kidney.
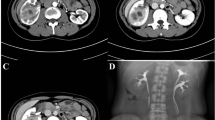
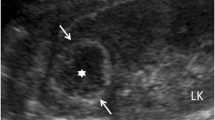
Abdominal ultrasound demonstrated a bilateral renal enlargement with multiple diffuse thin-walled cystic masses of various sizes throughout both kidneys, as well as a 7.7 cm × 6.0 cm × 5.7 cm cystic mass thickened walls and irregular septum in the middle and the lower pole of the right kidney (Fig. 1). After CEUS revealed the thickened walls and irregular septations in the cystic mass, there were unequivocal enhancements in the cortical phase, and isoenhancement in cortico-medullary and late phases, the non-enhanced cystic portions of the mass were anechoic (Fig. 2). Thus, the diagnosis of cystic renal cell carcinoma with ADPKD was made. The findings were consistent with contrast-enhanced CT and 18F FDG PET/CT. Contrast-enhanced CT images of the lower abdomen showing the enlarged kidneys with multiple cystic low-density shadows of unequal size, there was a separated cystic shadow in the middle and the lower pole of the right kidney, and moderate enhancement of septation at the rims of the cystic shadow (Fig. 3), and axial 18F FDG PET/CT image of the lower abdomen showing the FDG-avid the thickened walls in the cystic mass with the FDG-avid post-peritoneum lymph nodes (Fig. 4).
Following an informed discussion, the patient proceeded with a laparoscopic-assisted right radical nephrectomy under general anesthesia to confirm the diagnosis.
Grossly, the right kidney was enlarged in which a large number of cysts and abscesses have replaced most of the normal kidney tissue (Fig. 5A). Histopathological examination of the resected specimen revealed granulomatous inflammation with numerous foamy histiocytes, necrotic tissue, neutrophils, lymphocytes, and plasma cell infiltrates (Fig. 5B). These findings were in accordance with XGP complicated by ADPKD.
No intraoperative or postoperative complications were observed. Antibiotic treatment with intravenous etimicin sulfate was initiated for 6 days after surgery(dose 100 mg intravenously). At six months follow-up, the disease resolved well without any right flank pain, weakness, or low-grade fever, and the BUN and creatinine levels stabilized.
Discussion and conclusions
XGP is a rare and distinct form of chronic pyelonephritis that usually occurs in the background of a urinary tract obstruction caused by renal calculi and recurrent chronic infections [5]. Its most significant histological feature is the existence of lipid-laden foamy macrophages [6]. XGP can occur at any age, but has the highest incidence in immunocompromised middle-aged women. XPG’s pathology usually presents unilaterally, but occasionally bilaterally in the kidneys. The latter prognosis is worse than the former and is likely due to bilateral renal function impairment [7]. There are usually no characteristic symptoms of XGP.
In our case, there was a history of flank pain, weakness, low-grade fever, urinary tract infections, absence of dysuria, weight loss, nephrolithiasis, renal angle tenderness, and a palpable lump. However, renal complications of ADPKD can also include nephrolithiasis, infection, and pain [8]. Therefore, the preoperative diagnosis of XGP with ADPKD is challenging. This is an extremely rare case reporting XGP with ADPKD.
Imaging helped with the preoperative diagnosis of XGP. According to the imaging, XGP can be divided into two anatomic forms: the diffuse form and the focal form [9]. The diffuse form is the most common. The typical imaging signature is the “bear paw sign,”in which multiple rounded foci filled with lipid-laden macrophages dilated the renal pelvis and calices [10]. Our case did not observe the bear paw sign. Depending on the inflammation’s progression, XGP starts in the renal parenchyma and the perirenal adipose space and subsequently spreads to adjacent structures or the retroperitoneum, culminating in the formation of renoduodenal fistula, pancreatic adhesions or nephrocutaneous fistula [11,12,13]. Preoperative imaging should thoroughly evaluate the relationship of kidneys to the surrounding tissue and organs in order to prevent serious complications [14].
XPG has a close resemblance, clinical symptoms, and imaging findings to that of a renal tumor in the focal form, also known as the pseudotumor form [15]. Ultrasonic manifestations of renal cell carcinoma are round or oval solid space-occupying lesions, with complex internal echoes, but generally smooth edges and clear boundaries, and some of them can see low vocal cords. The focal form of XGP is a chronic inflammation, and the boundary of the lesions is vague. It can be preliminarily identified in combination with signs such as renal calculi and perirenal involvement.
In this case, the initial presumptive diagnosis was a cystic renal cell carcinoma with polycystic kidney disease. These results raised concerns about renal malignancies, including unequivocal enhanced masses in the right kidney imaged using CEUS and contrast-enhanced CT,the FDG-avid revealed the thickened walls in the cystic mass with the FDG-avid post-peritoneum lymph nodes imaged via 18F-FDG PET/CT. Meanwhile, this patient had a history of bilateral ADPKD, which further increased the preoperative diagnostic difficulty. However, if the focal XPG combined with ADPKD is misdiagnosed as a malignant renal tumor, it will lead to an unnecessary surgical resection. For focal XPG combined with ADPKD, especially patients with severe renal insufficiency or a solitary kidney we advocate an ultrasound-guided puncture biopsy of the focal mass in order to make a clear diagnosis before operation[16,17,18].
Beyond that, XGP might also mimic other renal tumors, renal tuberculosis, and renal abscesses. Nevertheless, lipid-laden foamy macrophages are typical and necessary for the diagnosis of XGP, and can be histopathologically differentiated from other renal diseases.
The recommended treatment of XGP includes surgery and antibiotic therapy. Surgery includes both an open and laparoscopic nephrectomy. Asali et al. [19] concluded that laparoscopic nephrectomy should be considered an initial approach for XGP. The indications for laparoscopic nephrectomy should be extended to these patients. Antibiotic therapy can serve as an alternative therapy for small and focal lesions.
In conclusion, XGP with polycystic kidney disease is extremely rare. The purpose of this case study is to emphasize that XPG should be taken into consideration in the differential diagnosis of renal masses with polycystic kidney disease, even in the absence of characteristic clinical symptoms. Careful preoperative examination plays an important role in choosing the treatment direction and operation mode.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- XGP:
-
Xanthogranulomatous pyelonephritis
- ADPKD:
-
Autosomal dominant polycystic kidney disease
- CT:
-
Computed tomography
- 18F FDG PET/CT:
-
Fluorine 18 fluorodeoxyglucose PET/CT
- CEUS:
-
Contrast-enhanced ultrasound
References
Schlagenhaufer F. Uber eigentumliche staphylomykosen der nieren und des pararenalen bindegewebes frankfurter. Z Pathol. 1916;19:139–48.
Ostalska-Nowicka D, Mackowiak-Lewandowicz K, Konwerska A, Zachwieja J. Early progression of xanthogranulomatous pyelonephritis in children might be dependent on vimentin expression. Am J Case Rep. 2017;18:1066–72.
Marinacci LX, Rosales I. Xanthogranulomatous pyelonephritis. N Engl J Med. 2018;378:940.
Xie X, Wang N, Wang Y, He H, Kong F, Li N. Non-invasive papillary urothelial carcinoma, low-grade of the renal pelvis mimicking a xanthogranulomatous pyelonephritis in a male patient: a case report and review of literature. Int J Immunopathol Pharmacol. 2020;34:2058738420925720.
Ding X, Wang G, Wang T, Ma X, Wang Y. Atypical focal xanthogranulomatous pyelonephritis without clinical symptoms presenting as infiltrative renal cancer: a case report and literature review. BMC Urol. 2020;20:63.
Kundu R, Baliyan A, Dhingra H, Bhalla V, Punia RS. Clinicopathological spectrum of xanthogranulomatous pyelonephritis. Indian J Nephrol. 2019;29:111–5.
Bansal K, Sureka B, Jain V, Arora A. Bilateral xanthogranulomatous pyelonephritis: morphologically rotund, functionally lame. Indian J Nephrol. 2015;25:255–6.
Ghata J, Cowley BD Jr. Polycystic kidney disease. Compr Physiol. 2017;7:945–75.
Chlif M, Chakroun M, Ben Rhouma S, Ben Chehida MA, Sellami A, Gargouri MM, Nouira Y. Xanthogranulomatous pyelonephritis presenting as a pseudotumour. Can Urol Assoc J. 2016;10:E36–40.
Garrido-Abad P, Rodríguez-Cabello MÁ, Vera-Berón R, Platas-Sancho A. Bear paw sign: xanthogranulomatous pyelonephritis. J Radiol Case Rep. 2018;12:18–24.
Holton-Burke RC, Varughese M. A case of xanthogranulomatous pyelonephritis associated with renoduodenal fistula. Case Rep Med. 2017;2017:8069205.
Devrim T, Atasoy P, Tuğlu D. Xanthogranulomatous pyelonephritis: a case with rare adhesion to pancreas. CEN Case Rep. 2018;7:44–7.
Weissman S, Ghaffar M, Safavian D, Rubal S, Khabut A, Maruf MG, Krzyzak M. Nephrocutaneous fistula due to xanthogranulomatous pyelonephritis. Cureus. 2018;10:e3467.
Kisa E, Keskin MZ, Yucel C, Yalbuzdag ON, Karabıcak M, Ilbey YO. Effect of kidney volume on the results of nephrectomy performed for xanthogranulomatous pyelonephritis. Cureus. 2019;11:e3976.
Tüysüz G, Tayfun F, Canpolat F, Zeytun H, Goya C, Keleş AN, Özdemir N. A case of xanthogranulomatous pyelonephritis mimicking Wilms tumor. Turk J Pediatr. 2015;57:409–12.
Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, Clark PE, Davis BJ, Derweesh IH, Giambarresi L, Gervais DA, Hu SL, Lane BR, Leibovich BC, Pierorazio PM. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–9.
Reul O, Waltregny D, Boverie J, de Leval J, Andrianne R. Pseudotumoral xanthogranulomatous pyelonephritis: diagnosis with percutaneous biopsy and success of conservative treatment. Prog Urol. 2001;11:1274–6.
Hunter RW, Ramaswamy R, Patel D, Dhaun N. Ultrasound-guided renal biopsy. Br J Hosp Med. 2017;78:C56–9.
Asali M, Tsivian A. Laparoscopic nephrectomy in xanthogranulomatous pyelonephritis. Cent European J Urol. 2019;72:319–23.
Acknowledgements
We thank the patient for allowing us to share his case.
Funding
Supported by Beijing Hospitals Authority Clinical Medicine Development of special funding support, No. XMLX202113; Beijing Municipal Science &Technology Commission, No. Z191100006619051; Special Fund for Open Topics of Lymph Surgery in Beijing Shijitan Hospital Affiliated to Capital Medical University, No. 2019-LB03.
Author information
Authors and Affiliations
Contributions
YL: designed the report; MY, QC: collected the patient’s clinical data; YL, MY: image analysis and manuscript writing; YL: finally reviewed the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and the accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yi, M., Liu, Y. & Chen, Q. Xanthogranulomatous pyelonephritis with polycystic kidney disease as a mimic of cystic renal cell carcinoma: a case report. BMC Urol 23, 58 (2023). https://doi.org/10.1186/s12894-023-01224-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-023-01224-7