Abstract
Background
Perineal ultrasound as a non-invasive method for the diagnosis of female stress urinary incontinence has attracted more and more attention. However, the criteria for stress urinary incontinence in women using perineal ultrasound have not been fully established. Our study aimed to evaluate characteristics of the urethral spatial movement with perineal ultrasonography.
Methods
A total of 136 female patients with stress urinary incontinence and 44 controls were enrolled. Stress urinary incontinence was diagnosed using the International Consultation on Incontinence Questionnaire Short Form, medical history and physical examination, and severity was assessed using a 1 h pad test. We described the mobility of four equidistant points (A–D) located along the urethra length. The retrovesical and urethral rotation angles were measured using perineal ultrasonography at rest and during the maximal Valsalva maneuver.
Results
Patients with stress urinary incontinence showed a more significant vertical movement at Points A, B and C than controls. The mean variations in the retrovesical angle were significantly larger in patients with stress urinary incontinence at rest and during the Valsalva maneuver than in controls (21.0 ± 16.5° vs. 14.7 ± 20.1°, respectively). The cut-off value for the retrovesical angle variation was 10.7° with 72% sensitivity and 54% specificity. There was a receiver-operating characteristic curve area of 0.73 and 0.72 for Points A and B, respectively. A cut-off of 10.8 mm, and 9.4 mm provided 71% sensitivity and 68% specificity and 67% sensitivity and 75% specificity, respectively.
Conclusions
The spatial movement of the bladder neck and proximal urethra, and variations in the retrovesical angle may be correlated with clinical symptoms and facilitate to the assessment of SUI.
Similar content being viewed by others
Introduction
The International Continmence Society (ICS) defines urinary incontinence (UI) as “any involuntary leakage of urine” or “urine leakage seen during examination”, that bladder pressure exceeds the pressure of the urethra has the capacity to closed [1]. Nearly half of adult women experience UI [2]. One study reported that the incidence of UI in women increases from 20.5% at age 15 to 68.8% at menopause [3]. The main types of UI include stress (SUI) and urge (UUI) urinary incontinence. SUI is the most prevalent type, especially in younger women [4, 5]. Approximately 6.8 million women had a primary diagnosis or chief complaint of UI in the United States in 2009/2010 [6]. Only 25% of incontinent women seek help and less than half receive treatment. [7]
Conventional diagnostic methods, including medical history and physical examination, UI questionnaires, urinalysis, and Q-tip testing, have certain limitations. Therefore, highly sensitive and specific tools are crucial for the correct diagnosis of UI. Loss of urethra function and morphological changes are important in the occurrence of urinary incontinence. Perineal ultrasonography (US) is a non-invasive test that depicts anatomical structures of the bladder and urethra and identifies urine leakage [8]. However, in previous studies, the indexes of urethral mobility may vary due to the difference of measurement methods and conditions [9,10,11,12,10], while every method has its emphasis.
In this study, perineal US were applied to focus on evaluate the two dimensions movement feature of the urethra between SUI and NSUI, meanwhile to assess the predictive value by the measurement method in female patients with SUI.
Methods
We registered 144 female patients with SUI and 50 controls between October 2020 and August 2021 in Chinese population. All patients provided informed consent and this analysis was approved by Yancheng third people’s hospital Human Research Ethics Committee. Assessments included medical history, physical examination, 3-day bladder diary, International Consultation on Incontinence Questionnaire Short Form (ICIQ-SF), urine routine, and biochemical examination. Patients with neuropsychiatric disorders, cognitive disorders, diabetes mellitus, urogenital surgery, pelvic irradiation, genitourinary tumors, pelvic organ prolapse, and urinary tract infections were excluded. The control group was women without pelvic disease and urinary incontinence during the same period. Finally, 136 female patients with SUI (SUI group) and 44 controls (NSUI group) were enrolled. Both groups underwent perineal US, and the SUI group additionally underwent 1 h pad test.
Perineal US
Perineal US was performed using the Voluson E8 system (GE Healthcare, Milwaukee, WI, USA) equipped with a 1–5 MHz curved array transducer placed on the perineum in a sagittal direction. And Residual urine volume should not exceed 100 ml. Using a coordinate system based on the origin of the lower margin of the symphysis pubis, manual measurements were obtained using the GE Kretz 4D View version 5.0 (GE Kretz, Zipf, Austria). US data were obtained at rest and during at least three Valsalva maneuvers held for 5–6 s, with the most effective measurement used for analysis. To determine the urethral motion profile, the urethral length was manually traced on US images in the mid-sagittal plane, both at rest and during the maximal Valsalva maneuver. This traced length was then divided into three segments with four equidistant points (A, B, C, D), from Point A (bladder neck) to Point D (external urethral meatus), as shown in Fig. 1. Mobility vector distances (MVD) for these points (AMVD, BMVD, CMVD, DMVD) were determined using the formula, \(\sqrt{{(Vx-Rx)}^{2}+({Vy-Ry)}^{2}}\) which allowed us to describe the difference between point coordinates from the lower margin of the symphysis pubis (x = horizontal distance; y = vertical distance) at rest (R) and during the Valsalva maneuver (V). The retrovesical angle (RVA; the angle formed by the urethral axis and a line drawn tangentially to the posterior edge of the bladder base near the bladder neck) and the urethral inclination angle (the angle between the urethral axis and the vertical axis) were measured both at rest and during the Valsalva maneuver. The urethral rotation angle (URA), which is the difference in urethral inclination angles at rest and during the Valsalva maneuver, was also measured (Figs. 1, 2). Image analysis: The images were analyzed by 2 physicians with at least 2 years of experience in real-time ultrasound and pelvic floor ultrasound, and the results were determined by consultation of the 2 physicians.
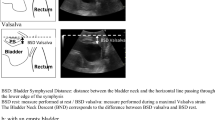
The urethra image was measured by perineal ultrasound image in the mid-sagittal plane at rest (left) and on Valsalva (right). The urethra is divided into three segments, defined by four points from the bladder neck (point A) to the external meatus (point D). The inferoposterior margin of the symphysis pubis serves as reference. The motion of Point A is shown as an example. AR was the position of point A at rest, and AV was the position of point A on maximal Valsalva manoeuver
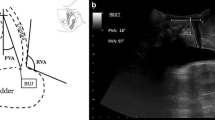
Perineal ultrasoubd image of the pelvic organs. Anatomical landmarks are the pubic symphysis (PS) and bladder with bladder neck (BN). The urethra (U). The posterior urethrovesical angle (the red arrow) and urethral inclination angle (the yellow arrow) was measured both at rest and on maximal Valsalva manoeuver
Statistical analyses
Statistical evaluation was performed using SPSS 21 for Windows (SPSS, Chicago, IL, USA). Data are presented as mean ± standard deviation. The results of perineal ultrasonography in our study were compared using the paired t-test for normally distributed data and the Wilcoxon test for non-normally distributed data. Two-tailed P < 0.05 was considered statistically significant. We constructed receiver-operator characteristic (ROC) curves to choose cut-off limits of urethral mobility to discriminate between normal and incontinent women.
Results
The mean age in the SUI and NSUI groups was 54.4 ± 9.5 (range, 29–78) and 49.3 ± 10.4 (range, 29–70) years, respectively. Seventy-five percent of women with SUI were vaginally parous [mean parity 2.1 ± 1.4 (range: 1–7)], while 68% of NSUI women were vaginally parous [2.0 ± 1.5 (0–5)]. Body mass index (BMI) was 22.5 ± 3.4 (range: 17.3–29.7 kg/m2) in the SUI group and 22.4 ± 3.1 (17.5–30.2) in the NSUI group (Table 1).
Based on the 1-h pad test, 73 patients had moderate to severe SUI (> 10 g) and 63 patients had mild SUI (< 10 g). In the moderate to severe SUI group, the mean age was 54 ± 9.4 (33–75) years, mean BMI was 22.7 ± 3.6 (17.8–28.8 kg/m2), mean vaginal parity was 2.1 ± 1.4 (1–7), and mean ICIQ-SF score was 11.9 ± 4.8 (0–21). In the mild SUI group, the mean age was 55 ± 9.7 (29–78) years, mean BMI was 22.6 ± 3.2 (17.3–29.7 kg/m2), mean vaginal parity was 2.1 ± 1.4 (1–7), and mean ICIQ-SF score was 5.9 ± 6.0 (0–17). On the 1 h standardized pad test, women from the moderate to severe group leaked more urine than those from the mild group [30.3 ± 24.3 (10.8–82 g) vs. 3.0 ± 2.4 (0.27–8.7 g); P < 0.05] (Table 2).
The mean URA was 25.3 ± 18.4° (1–69.0°) in the SUI group and 26.3 ± 18.5° (1–67.8°) in the NSUI group (P > 0.05). The mean variation in RVA (RVAδ) was significantly higher in the SUI group [21.0 ± 16.5° (−28.5 to 63.5°) vs. 14.7 ± 20.1° (−30.9 to 60.9°); P < 0.05] (Table 3).
Perineal US measurements of the mean mobility of the segmental urethra from the bladder neck (A) to the external urethral orifice (D) are shown in Table 5. The mean mobility of the bladder neck (Point A) was 14.6 ± 7.0 (2.0–33.4) mm in the SUI group and 10.0 ± 4.1 (2.0–22.3) mm in the NSUI group. The same measurements at Points B–D were 11.9 ± 5.6 (3.9–27.5), 8.5 ± 4.9 (0.4–22.5), and 6.8 ± 3.7 (1.3–21.0) mm, respectively, in the SUI group and 8.2 ± 3.4 (2.9–20.0), 7.1 ± 2.7 (0.3–13.9), and 6.1 ± 3.5 (2.2–23.0) mm, respectively, in the NSUI group. There was a generalized change of segmental urethral mobility in the SUI group; however, this only reached significance (vs. NSUI) at Points A, B and C. (Table 4).
Analysis of the characteristics of Points A, B and C in the two groups showed significant differences in the mean mobility vectors at Point A in the horizontal and vertical direction [9.5 ± 6.3 (SUI) and 6.8 ± 3.9 mm (NSUI); 9.8 ± 1.2 (SUI) and 6.1 ± 3.4 mm (NSUI), respectively, both P < 0.05] and at Point B in the horizontal and vertical direction [6.8 ± 4.0 (SUI) and 5.3 ± 2.9 (NSUI), P > 0.05; 8.9 ± 5.4 (SUI) and 5.3 ± 3.3 (NSUI), P < 0.05] and at Point C in the horizontal and vertical direction [3.8 ± 3.0 (SUI) and 4.0 ± 2.6 (NSUI), P > 0.05; 7.3 ± 4.9 (SUI) and 5.1 ± 3.2 (NSUI), P < 0.05] (Table 5).
ROC curves for RVA and segmental urethral mobility (Points A and B) were constructed to determine the cut-off values for SUI and NSUI discrimination. In the RVAδ examination, we obtained an ROC area of 0.62 [95% confidence interval (CI) 0.52–0.72] and chose 10.7° as the cut-off point with 72% sensitivity and 54% specificity. Segmental urethral mobility examination resulted in an ROC area of 0.73 (95% CI 0.66–0.81) for Point A, and a cut-off of 10.8 mm provided 71% sensitivity and 68%, specificity. We obtained an ROC area of 0.72 (95% CI 0.76–0.94) for Point B and chose 9.4 mm as the cut-off point with 67% sensitivity and 75% specificity (Fig. 3).
ROC curves (receiver operating curve) analyses of three parameters for predicting stress urinary incontinence by perineal ultrasonography. Point A: the mobility vector distances of A (AMVD); Point B: the mobility vector distances of B (BMVD); RVAδ: the mean variation of retrovesical angle on rest and most effectively Valsalva
Discussion
In this study, we explored the meaning of segmental urethral mobility of the urethra by perineal US. We found that the mobility in all urethral segments, especially the proximal urethra, along with the RVAδ played a significant role in the pathogenesis of SUI.
Sendag [13] reported that a descending distance of the bladder neck > 15 mm and a posterior RVA > 120° were related to poor support of the bladder neck in patients with SUI with 96% and 53% sensitivity and 85% and 100% specificity, respectively. During straining condition, SUI women demonstrated > 1 cm of caudal motion of their urethrovesical junction by transrectal US, while control had < 1 cm of that [14]. Another study speculated that a bladder neck distance (BND) ≥ 25 mm may be abnormal and associated with SUI [15]. Our results showed that the sensitivity and specificity of cut-off point A (bladder neck > 10.8 mm) as a diagnostic criterion were 71% and 68%, respectively. The inter-study differences in the results related to the descending distance of the bladder neck may be due to different measurement methods, as well as race. We combined the vertical and horizontal motion of the bladder neck, which may provide a better representation of the spatial movement in the bladder neck. However, a recent study confirmed that BND alone cannot comprehensively evaluate SUI [16]. Similar to our results, one Chinese report suggested that the specificity of BND in SUI diagnosis was only 68.9% [17]. Thus, we need to explore the abnormal urethral support structure to understand the etiology and pathogenesis of SUI. Most studies have focused on bladder neck mobility and provided little information about the rest of the urethra [18]
The phenomenon where there is a rotational movement around the tip of the symphysis pubis during stress was observed on US in all patients. This rotational movement occurs in a cranial-to-caudal and dorsal-to-ventral direction, which is why we decided to use a mathematical description of urethral mobility. Our results showed that this change in mobility was similar for all urethral segments and was not restricted to the mid-urethra, suggesting that mobility at all stages of the urethra should be considered in UI studies. One previous study divided the urethra into five equal segments with six points from the bladder neck to the external urethral meatus, and results showed that the proximal urethra was consistently more mobile than the distal urethra (P < 0.001) [19]. However, they did not compare horizontal movement. In our study, the mean mobility and vertical distance of Points A (YA), B (YB) and C(Yc) in SUI were much greater than those in NSUI, and horizontal movement was one of the most influential factors. The horizontal distance of Point A in SUI was significantly greater than that in NSUI. However, there was no significant difference between SUI and NSUI at Point B and C. We speculated that there is more horizontal and vertical movement of the urethra closer to the neck of the bladder. Points D were the least mobile, suggesting that loss of proximal urethral stability leads to abnormalities in urethral mobility, resulting in SUI.
SUI is related to both urethral support and morphological changes in the junction of the urethra and bladder. In this study, there was no obvious change in urethral inclination, but the mean RVAδ at rest and during the Valsalva maneuver was clearly different in SUI and NSUI patients. A similar study showed that SUI was significantly associated with RVAδ. The mean RVA during maximum Valsalva was 152°, which was similar to our results (most effective Valsalva maneuver 143.4 ± 15.7°). We believe that the difference in RVA may be related to bladder volume and Valsalva maneuvers during detection. We further tested the mean RVAδ at rest and during the Valsalva maneuver, and speculated that this indicator may better explain the differences in bladder volumes. However, a previous study confirmed that there are no differences in BND, URA, or posterior urethro-vesical angle (PUVA) for different bladder capacities (100–500 ml) [20]. One study [21] revealed that BND, URA, and RVA were not significantly different in mild to moderate and severe patients. Thus, perineal US is a useful tool for identifying SUI, but not its severity. Real time assessment of bladder neck and proximal urethra behavior using transperineal US indicated that the median RVA (166°) in the standing position was significantly greater than RVA (133°) in the supine position, and the median URA of 35° was significantly smaller than that of 64° in the supine position [22]. In our study, the measurements were performed with patients in the supine position, and the mean URA of 25.3 ± 18.4° and mean RVA of 143.4 ± 15.7° were smaller than reported by the previously mentioned study. This may be due to different patient variables and the standard of Valsalva maneuvers. We found no obvious difference in RVA at rest between SUI and NSUI, but there was a significant difference in RVA during the Valsalva maneuver. Another report showed similar results whereby the difference in PUVA at rest and during Valsalva maneuver in SUI was significantly higher than in NSUI [23].
In terms of BND results (optimal cut-off, 24 mm), the area under the ROC curve (AUC) value was 0.804, with 66.4% sensitivity and 84.5% specificity24. In our results, the ROC area of Point A was 0.73 with the optimal cut-off 10.8 mm, and this cut-off point provided 71% sensitivity and 68%, specificity. Moreover, Point B (cut-off 9.4 mm) provided 67% sensitivity and 75% specificity. We believe that bladder neck activity and the first one-third of the length from the urethra to the bladder neck has value in predicting UI. The AUC value of RVAδ was 0.62 (cut-off 10.7°), with 72% sensitivity and 54% specificity. This represents a weak predictive index for SUI.
Conclusions
In our study, the horizontal and vertical direction movement of proximal urethra and RVAδ were the variables most closely associated with SUI. These parameters may be correlated with clinical symptoms and facilitate to the assessment of SUI.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. The images in study were appropriated permission by patients.
Abbreviations
- AUC:
-
Area under the ROC curve
- BMI:
-
Body mass index
- BND:
-
Bladder neck distance
- ICIQ-SF:
-
International Incontinence Questionnaire Short Form
- ICS:
-
International Continence Society
- MVD:
-
Mobility vector distances
- PUVA:
-
Posterior urethro-vesical angle
- ROC:
-
Receiver-operator characteristic
- RVA:
-
Retrovesical angle
- RVAδ:
-
Mean variation in RVA
- UDS:
-
Urodynamic study
- UI:
-
Urinary incontinence
- URA:
-
Urethral rotation angle
- US:
-
Ultrasonography
References
D’Ancona C, Haylen B, Oelke M, Abranches-Monteiro L, Arnold E, Goldman H, et al. The international continence society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn. 2019;38(2):433–77.
Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstet Gynecol. 2008;111:324.
Topazio L, Frey J, Iacovelli V, et al. Prevalence of “complicated” stress urinary incontinence in female patients: can urodynamics provide more information in such patients? Int Urogynecol J. 2015;26:1333.
Holroyd-Leduc JM, Tannenbaum C, Thorpe KE, et al. What type of urinary incontinence does this woman have? JAMA. 2008;299:1446.
Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc. 1998;46:473.
Forde JC, Chigtai B, Cea M, et al. Trends in ambulatory management of urinary incontinence in women in the United States. Female Pelvic Med Reconstr Surg. 2017;23:250.
Minassian VA, Yan X, Lichtenfeld MJ, et al. The iceberg of health care utilization in women with urinary incontinence. Int Urogynecol J. 2012;23:1087.
Jamard E, Blouet M, Thubert T, Rejano-Campo M, Fauvet R, Pizzoferrato AC. Utility of 2D-ultrasound in pelvic floor muscle contraction and bladder neck mobility assessment in women with urinary incontinence. J Gynecol Obstet Hum Reprod. 2020;49(1):101629.
Falah-Hassani K, Reeves J, Shiri R, Hickling D, McLean L. The pathophysiology of stress urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2021;32(3):501–52.
Li YQ, Geng J, Tan C, Tang J, Yang X. Diagnosis and classification of female stress urinary incontinence by transperineal twodimensional ultrasound. Technol Health Care. 2017;25(5):859–66.
Lukanovic A, Patrelli TS. Validation of ultrasound scan in the diagnosis of female stress urinary incontinence. Clin Exp Obstet Gynecol. 2011;38(4):373–8.
Najjari L, Janetzki N, Kennes L, Stickeler E, Serno J, Behrendt J. Comparison ofperineal Sonographically measured and functional urodynamic urethral length in female urinary incontinence. Biomed Res Int. 2016;2016:4953091.
Sendag F, Vidinli H, Kazandi M, et al. Role of perineal sonography in the evaluation of patients with stress urinary incontinence. Aust N Z J Obstet Gynaecol. 2003;43:54.
Bergman A, Ballard CA, Platt LD. Ultrasonic evaluation of urethrovesical junction in women with stress urinary incontinence. J Clin Ultrasound. 1988;16(5):295–300.
Naranjo-Ortiz C, Shek KL, Martin AJ, et al. What is normal bladder neck anatomy? Int Urogynecol J. 2016;27:945.
Dietz HP, Kamisan Atan I, Salita A. Association between ICS POP-Q coordinates and translabial ultrasound findings: implications for definition of “normal pelvic organ support.” Ultrasound Obstet Gynecol. 2016;47:363.
Lu R, Zhang Y, Dai FR, et al. Application of transperineal pelvic floor ultrasonography in the diagnosis of female stress urinary incontinence. Natl Med J China. 2018;98:2675.
Pirpiris A, Shek KL, Dietz HP. Urethral mobility and urinary incontinence. Ultrasound Obstet Gynecol. 2010;36:507.
Shek KL, Dietz HP. The urethral motion profile: a novel method to evaluate urethral support and mobility. Aust N Z J Obstet Gynaecol. 2008;48:337.
Zhi-qin C, Yan-feng S, Jian-ping Z. Significance of perineal sonography of different bladder capacity in the diagnosis of women with stress urinary incontinence. Chinese J Pract Gynecol Obstet. 2008;24:596.
Li YQ, Geng J, Tan C, et al. Diagnosis and classification of female stress urinary incontinence by transperineal two-dimensional ultrasound. Technol Health Care. 2017;25:859.
Wen L, Zhao B, Chen W, et al. Real-time assessment of the behaviour of the bladder neck and proximal urethra during urine leaking in the cough stress test (CST) in supine and standing positions using transperineal ultrasound. Int Urogynecol J. 2020;31:2515.
Al-Saadi WI. Transperineal ultrasonography in stress urinary incontinence: the significance of urethral rotation angles. Arab J Urol. 2016;14:66.
Xiao T, Chen Y, Gan Y, et al. Can stress urinary incontinence be predicted by ultrasound? AJR Am J Roentgenol. 2019;213:1163.
Acknowledgements
We thank Elsevier Language Editing Services for his valuable comments on draft manuscripts.
Funding
Medical Science and Technology Development Plan project of Yancheng (YK 2019099). Clinical Medicine Program of Nantong University (2019LY 012).
Author information
Authors and Affiliations
Contributions
BD: operation/ writing/data collection; YS: operation/data collection; YC: operation/data collection; ML: operation; XL: editing; YL: design/writing/operation/data collection. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Our study has been approved by the Institutional review board ethics committee and all persons provided their informed consent prior to their inclusion in the study. All patients provided informed consent and this analysis was approved by Yancheng third people’s hospital Human Research Ethics Committee (number: 2020-22 [2]). All methods were performed in accordance with the relevant guidelines and regulations.
Consent to publish
All authors provided consent for publication. Written informed consent was obtained from the patient in the study for publication of their individual details and any accompanying images in this manuscript.
Competing interests
All authors declare that they have no conflicts of interest regarding a financial relationship with the organization that sponsored the research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, B., Shi, Y., Chen, Y. et al. Perineal ultrasound to assess the urethral spatial movement in stress urinary incontinence in women. BMC Urol 23, 44 (2023). https://doi.org/10.1186/s12894-023-01220-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-023-01220-x