Abstract
Background
Drainage is indicated in many patients with a perinephric abscess (PA). Surgical drainage is associated with trauma and slow recovery, while percutaneous drainage can be ineffective in some patients. We report on 11 patients with PA treated by percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction under local anesthesia.
Methods
This case series included 11 PA patients operated on from January 2013 to June 2020. All patients received percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction. Data, including operation time, volume of intraoperative blood loss, volume of intraoperative pus suction, time of postoperative drainage tube indwelling, time to restore normal body temperature, length of postoperative hospital stay, and intraoperative and postoperative complications, were collected.
Results
The age of the patients was 59 (53–69) years. Eight, six, two, and two patients had hypertension, type 2 diabetes, rheumatoid arthritis, and renal calculi, respectively. The operations were successful forall11 patients. Eight, two, and one patients required one, two, and three channels, respectively, to clear their abscess. The average operation time was 44 (30–65) min, and intraoperative blood loss was 16 (10–20) ml. The volume of intraoperative pus suction was 280 (200–400) ml, time of postoperative drainage tube indwelling was 8.2 (6–12) days, and time to restoring normal body temperature was 0.8 (0.5–2) days. The average postoperative hospital stay was 9.8 (7–14) days. No severe intraoperative or postoperative complications occurred. The postoperative follow-up time was typically 4.8 (3–8) months, and there were no recurrences.
Conclusion
Percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction might be a feasible method for treating PA.
Similar content being viewed by others
Background
A complicated urinary tract infection is an infection of either the upper or lower urinary tract in a patient at an increased risk of treatment failure or complications [1, 2]. Complications can be systemic (e.g., sepsis) or local (e.g., renal abscess, perinephric abscess [PA], and papillary necrosis). PA is formed by the spread of a pyogenic infection into the adipose tissues between the renal capsule and perirenal fascia [3, 4], and the risk factors include diabetes, immunosuppression, pregnancy, neurogenic bladder, nephrolithiasis, indwelling urinary devices, and urinary obstruction [1, 2, 5]. The incidence of renal and PA in people with diabetes is 46 per 100,000 person-year, compared with 11 per 100,000 person-year in non-diabetic controls [6]. In addition, 20%-60% of patients with PA have associated renal calculi [4]. Escherichia coli is the most common cause of PA, followed by other Enterobacteriaceae (such as Klebsiellasp., Proteussp., and Serratiasp.), as well as Pseudomonas sp. and Enterococcussp. [1, 3, 7,8,9].
Due to the non-specificity and complexity of PA, delayed diagnosis, and the limitations of the available treatments, the mortality rate of patients with PA used to be as high as 40–50% [9].
With the continuous advancement of medical techniques, the diagnosis of PA is no longer difficult, and there are more choices regarding treatment. Conventional therapy includes antibiotics and control of the infection source, but it cannot control the space-occupying effect of the abscess, and surgery maybe indicated [1, 2, 7]. The failure of conservative treatment is also an indication for surgery [1, 2, 4, 7]. Conventional open PA incision and drainage and the more recent laparoscopic PA incision and drainage both require general anesthesia and involve surgical trauma, and patients recover slowly. Ultrasound- or computed tomography (CT)-guided percutaneous drainage can be performed but sometimes ineffective due to several disadvantages, including incomplete drainage and requiring repeated drainages, especially when treating large septal abscesses with thick pus [4, 10, 11]. Therefore, developing better treatments has become a hotspot for clinicians.
This study aimed to report on the details and outcomes of 11 patients with PA treated by percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction under local anesthesia. The results suggest this novel method could be used for the effective management of PA.
Methods
Study design and patients
This retrospective study included all patients with PA treated by percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction under local anesthesia from January 2013 to June 2020 in Zhejiang Provincial People’s Hospital. This study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (No. 2021QT030). The requirement for informed consent was waived by the committee due to the retrospective nature of the study.
Surgical methods
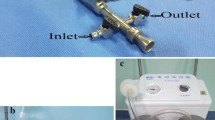
The patients were placed in the prone position with a pad under their waist. A puncture point was selected at the site where the abscess was closest to the body surface while avoiding the major blood vessels and vital organs (e.g., liver and spleen) according to intraoperative ultrasound (BK Ultrasound, Denmark) positioning. Local infiltration anesthesia using 15 ml of 2% lidocaine was performed. An 18 G puncture needle (Create Medic, Japan) was inserted to the clearest sonolucent area of the fluid in the abscess’s center under ultrasound guidance. A small amount of pus was collected for routine bacterial culture and drug sensitivity tests. A fascial dilator (Create Medic, Japan) was used to dilate gradually from 8 to 20 Fr under the guidance of a guidewire (Create Medic, Japan) and then a 20 Fr outer sheath (Create Medic, Japan) was placed. An 18 Fr nephroscope (Richard Wolf, Germany) was inserted through the sheath. Then, negative-pressure suction (EMS, Switzerland) was used to aspirate the pus and tissues completely, and foreign body forceps (Richard Wolf, Germany) were used to remove necrotic tissue in the abscess (Fig. 1). A continuous negative-pressure rinse was performed until the aspirated fluid was clear; then the nephroscope was withdrawn. An 18 Fr drainage tube with multiple lateral holes (Create Medic, Japan) was placed through the sheath, and the incision was sutured to fix the drainage tube. According to the intraoperative ultrasound findings, a second and sometimes third channel was established to further clear septal abscesses, and drainage tubes were placed in each channel. After the surgery, sensitive antibiotics were used for anti-infection therapy. Re-examination by urinary CT was performed when there was no evident pus in the drainage tube. If CT showed that the abscess had disappeared and there was no discomfort after clamping the drainage tube, the patients could remove the drainage tube and be discharged from hospital.
Data collection
Data, including operation time, volume of intraoperative blood loss, volume of intraoperative pus suction, time of postoperative drainage tube indwelling, time to restore normal body temperature, length of postoperative hospital stay, and intraoperative and postoperative complications were collected. The patients were followed up at the outpatient department, and any recurrences were monitored. Ultrasound examination was performed to monitor recurrences.
Statistical analysis
Only descriptive statistics were used. Continuous data are summarized using average (range). Categorical data are presented as n (%).
Results
Eleven patients with PA, including three males and eight females, were included in this study. Table 1 presents the characteristics of the patients. The average age of the patients was 59 (53–69) years. All patients had fever and waist pain; two patients had shivering; two had bladder irritation sign; and four had abdominal distension, nausea, and a poor appetite. The abscess was on the left and right side in four and seven patients, respectively. The average maximum diameter of the abscesses was 9.9 (8.1–12.2) cm. Eight, six, two, and two patients had hypertension, type 2 diabetes, rheumatoid arthritis, and renal calculi, respectively. In addition, two patients had secondary infection due to perirenal hematoma after flexible ureteroscopic lithotripsy.
Table 2 presents the characteristics of the operations. Operations for all 11 patients were successful. Eight, two, and one patients required one, two, and three channels, respectively, to clear their abscess. The average operation time was 44 (30–65) min. The average intraoperative blood loss volume was 16 (10–20) ml, and the average volume of intraoperative pus suction was 280 (200–400) ml. The average time of postoperative drainage tube indwelling was 8.2 (6–12) days, and the average time to restore normal body temperature was 0.8 (0.5–2) days. The average postoperative hospital stay was 9.8 (7–14) days. No severe intraoperative or postoperative complications occurred. The average postoperative follow-up time was 4.8 (3–8) months and there were no recurrences.
Discussion
Drainage is indicated in many patients with PA. Surgical drainage is associated with trauma and slow recovery, while percutaneous drainage can be ineffective in some patients [4, 10, 11]. Therefore, this case series reports the details of patients with PA who were treated by percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction under local anesthesia. The results suggested that percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction is feasible as a method for treating PA.
The risk factors for PA are diabetes, immunosuppression, pregnancy, neurogenic bladder, nephrolithiasis, indwelling urinary devices, and urinary obstruction [1, 2]. Among the 11 patients in this study, two patients had a clear history of upper respiratory tract infection before onset, considering that bacterial hematogenous infection led to the PA. Two patients had secondary infection due to perirenal hematoma after flexible ureteroscopic lithotripsy. The PA of the other seven patients was mainly considered to be caused by pyelonephritis due to the increase of white blood cells in urine. Which is worthy of our attention, with the wide application of flexible ureteroscopic lithotripsy, many patients have a perirenal hematoma after lithotripsy. The risk factors for perirenal hematoma include high intraoperative perfusion pressure, being female, advanced age, diabetes, hypertension, renal insufficiency, urinary infection, coagulation disorders, the use of antiplatelet drugs, large calculus, long operation time, infectious calculus, and upper urinary tract obstruction [12]. The relatively small perirenal hematoma without secondary infection could be gradually absorbed if there are no clinical symptoms. If the hematoma is followed by secondary infection and PA, the patients will develop a severe fever, and anti-infectious therapy can result in relatively poor effects, and thus, surgical interventions are generally required [10, 12].
It is generally considered that, for PA with diameter of < 3 cm with non-matured and liquified content, sufficient antibiotics should be applied in time as conservative systemic therapy [1, 2, 7]. For PA with diameter of 3–5 cm, on which the effects of simple anti-infectious therapies are not evident, drainage should be performed in time [1, 2, 4, 7]. For PA with diameter of > 5 cm, drainage should be performed as early as possible in addition to anti-infectious therapy [1, 2, 4, 7, 10]. Ultrasound- and CT-guided percutaneous drainage is simple, convenient, minimally invasive, inexpensive, and can be performed under local anesthesia [4, 10, 11]. Nevertheless, this method has several disadvantages, such as incomplete drainage, a high risk of drainage tube obstruction, the inability to drain abscess cavities with incomplete liquefaction or sticky pus, and the requirement for repeated punctures for septal abscesses [4, 10, 11]. Conventional open and laparoscopic PA incision and drainage methods have the advantages of complete drainage, the possibility of rinsing the abscess cavities, and suitability for treating relatively large abscesses, septal abscesses, multiple abscesses, and abscesses with thick pus. Despite this, these methods involve more significant trauma than percutaneous drainage, require general anesthesia, and the patients have a risk of complications, relatively slow recovery, and a longer hospital stay [10, 13]. In addition, laparoscopic surgery should be chosen with caution for patients with a long disease course and severe perinephric adhesions. Peritoneal injury, excessively high CO2 pressure, a long operation time, and waist myofascial injuries can spread the infection [13].
Rai et al. [14] and Ng et al. [15] reported that treating PA by percutaneous nephroscopy achieved a satisfactory outcome. In this study, further modifications to this method were explored. An ultrasound-guided perinephric puncture was performed under local anesthesia to establish a percutaneous nephroscopy channel. Then, negative-pressure suction was used to aspirate the pus and tissues, and forceps were used to remove the necrotic tissue. This method is applicable to all PA requiring surgical drainage.
The anesthesia methods for percutaneous nephroscopy are currently general anesthesia and combined spinal-epidural anesthesia [16]. In patients with severe infections, especially with renal insufficiency, the capabilities of water-electrolyte regulation and metabolite excretion are relatively poor, and the risk of general anesthesia is elevated [17, 18]. Pain during percutaneous nephroscopy is mainly from stimulation of the sensory somatic nerves and visceral sensory nerves. As the area of puncture is relatively small, local-infiltration anesthesia is enough to eliminate the pain conducted by the somatic nerves [19]. Advancements in techniques and improvements to equipment have made percutaneous nephroscopy feasible, with the advantages of lower and controllable pressure, a shorter operation time, and providing a safer surgical process. Percutaneous nephroscopy has already been performed under local anesthesia by several groups [19, 20] and could favor patients’ early and rapid recovery.
The establishment of a standard channel for percutaneous nephroscopy under local anesthesia could provide a good visual field while maintaining low perfusion and allowing the rapid and highly efficient clearing of pus by negative-pressure suction. For thick pus that cannot be easily aspirated, suction can be performed after repeated rinsing with normal saline. The locally necrotic tissues, organized blood clots, and pus moss can be aspirated after ultrasound disintegration or using foreign body forceps with nephroscopy. For septal abscess cavities, nephroscopy combined with ultrasound negative-pressure suction can be used to break the relatively thin septa under direct vision before aspirating the pus. For septal abscess cavities that cannot be reached through the first channel, multiple channels can be established under ultrasound guidance according to the sizes and ranges of the abscesses, but wide drainage tubes with multiple lateral holes have to be placed for each channel. For abscesses with liquefaction of necrotic tissues, a small amount of pus can still be drained from the drainage tubes after the operation. Thus, the drainage tube should be maintained in the patient, and sterilized normal saline could be used to rinse if necessary.
We summarize the key points of this operation as follows. First, the center of the abscess should be selected as accurately possible to avoid the pain induced by large angle swings of nephroscopy. Second, low-flow perfusion should be performed to reduce pain and water pressure during rinsing. The puncture must be precise to avoid excessive bleeding, which could lead to an unclear visual field. Third, continuous negative-pressure suction during the operation could reduce the pressure in the abscess cavity, decrease the risk of bacteria and toxin backflow into the blood, and induce fluid exudation, reducing the incidence of postoperative urinary sepsis. Finally, the sites and numbers of channels used for nephroscopy should be appropriately planned under the guidance of ultrasound, and wide drainage tubes with multiple lateral holes should be placed in each channel after the operation to help obtain the best pus-clearing and drainage effects with minimal trauma.
This study had some limitations. The patients were from a single center, and the sample size was small. In addition, only a short follow-up period was included. Prospective studies with a large sample size and long-term follow up are needed to verify our findings.
Conclusions
In conclusion, this study suggests that percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction might be feasible for treating PA. This method has the advantages of minimal invasion, complete drainage, rapid recovery, and fewer complications. Skillful operators could choose to apply this method.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PA::
-
Perinephric abscess
- UTI::
-
Urinary tract infection
- CT::
-
Computed tomography
References
Bonkat G, Bartoletti R, Bruyère F, Cai T, Geerlings SE, Koves B, et al. EAU Guidelines on urological infections. Arnhem: European Association of Urology; 2020.
Nicolle LE. Ammi Canada guidelines committee.* Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16:349–60.
Dembry LM, Andriole VT. Renal and perirenal abscesses. Infect Dis Clin North Am. 1997;11:663–80.
Okafor CN, Onyeaso EE. Perinephric abscess. Treasure Island: StatPearls; 2021.
Zhou J, Liang C, Ye Y. Treatment of severe post-ESWL complications. Zhong Hua Mi Niao Wai Ke Za Zhi. 2014;35:691–4.
Ko MC, Liu CC, Liu CK, Woung LC, Chen HF, Su HF, et al. Incidence of renal and perinephric abscess in diabetic patients: a population-based national study. Epidemiol Infect. 2011;139:229–35.
Bader MS, Hawboldt J, Brooks A. Management of complicated urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2010;122:7–15.
Levison ME, Kaye D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr Infect Dis Rep. 2013;15:109–15.
Coelho RF, Schneider-Monteiro ED, Mesquita JL, Mazzucchi E, Marmo Lucon A, Srougi M. Renal and perinephric abscesses: analysis of 65 consecutive cases. World J Surg. 2007;31:431–6.
Rubilotta E, Balzarro M, Lacola V, Sarti A, Porcaro AB, Artibani W. Current clinical management of renal and perinephric abscesses: a literature review. Urologia. 2014;81:144–7.
Gardiner RA, Gwynne RA, Roberts SA. Perinephric abscess. BJU Int. 2011;107(Suppl 3):20–3.
Whitehurst LA, Somani BK. Perirenal hematoma after ureteroscopy: a systematic review. J Endourol. 2017;31:438–45.
Hao C, Zhang N. Shan Z [Clinical analysis of the drainage of perinephric abscess with retroperitoneal laparoscopy]. Zhong Hua Mi Niao Wai Ke Za Zhi. 2014;35:258–61.
Rai RS, Karan SC, Kayastha A. Renal and perinephric abscesses revisited. Med J Armed Forces India. 2007;63:223–5.
Ng CF, Liong YV, Leong WS, Harris DF, Lau BE, Liong ML. A better way to manage perinephric abscesses: percutaneous ultrasonography-guided endoscopic lavage. J Endourol. 2014;28:528–31.
Hu H, Qin B, He D, Lu Y, Zhao Z, Zhang J, et al. Regional versus general anesthesia for percutaneous nephrolithotomy: a meta-analysis. PLoS ONE. 2015;10: e0126587.
Motayagheni N, Phan S, Eshraghi C, Nozari A, Atala A. A Review of anesthetic effects on renal function: potential organ protection. Am J Nephrol. 2017;46:380–9.
Wagener G, Brentjens TE. Anesthetic concerns in patients presenting with renal failure. Anesthesiol Clin. 2010;28:39–54.
Akman T, Binbay M, Aslan R, Yuruk E, Ozgor F, Tekinarslan E, et al. Long-term outcomes of percutaneous nephrolithotomy in 177 patients with chronic kidney disease: a single center experience. J Urol. 2012;187:173–7.
Chen Y, Zhou Z, Sun W, Zhao T, Wang H. Minimally invasive percutaneous nephrolithotomy under peritubal local infiltration anesthesia. World J Urol. 2011;29:773–7.
Acknowledgements
Not applicable.
Funding
This study was supported by the Major Science and Technology & Key Social Development Projects of Zhejiang Science and Technology Department [grant number 2014C03047–1]; and the General Research Project of Zhejiang Education Department [grant number Y202044661].
Author information
Authors and Affiliations
Contributions
EHL and WWY conceived the idea of the study; MZ and YLZ analysed the data; XH and DHZ contributed significantly to analysis and manuscript preparation; EHL wrote the paper; All authors discussed the results and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study was carried out in accordance with ethical guidelines of Zhejiang Provincial People’s Hospital affiliated to Hangzhou Medical College and approved by the Ethics Committee of Zhejiang Provincial People’s Hospital affiliated to Hangzhou Medical College (No. 2021QT030). The Ethics Committee of Zhejiang Provincial People’s Hospital affiliated to Hangzhou Medical College has waived the need of informed consent for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, E., Hong, J., Zhou, M. et al. Percutaneous nephroscopy combined with ultrasound-guided negative-pressure suction for the treatment of perinephric abscess: a case series. BMC Urol 22, 140 (2022). https://doi.org/10.1186/s12894-022-01091-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-022-01091-8