Abstract
Background
Multiple kidney tumours are frequently seen in hereditary syndromes and familial diseases. Renal collision tumours (RCT) are characterized by the simultaneous existence of different and unrelated tumour types within the same location in the kidney, forming a single, heterogenous lesion. RCT are uncommon histological entities with distinctive features. The most frequent subtypes include clear cell renal cell carcinoma (CCRCC), papillary renal cell carcinoma (PRCC), chromophobe renal cell carcinoma (CRCC), and collecting duct carcinoma (CDC).
Case presentation
Here, we report three sporadic cases of RCT successfully treated by nephrectomy and confirmed by histological analysis. The first case was of a 64-year-old man diagnosed with RCT composed of a stage 2 nucleolar grade 3 CCRCC and a stage 1a nucleolar grade 2 type 1 PRCC. The second case was of a 68-year-old woman diagnosed with a combined nucleolar grade 2 type 1 PRCC and an angiomyolipoma (non-assessed stage), while the third case was of a 59-year-old woman diagnosed with a combined stage 1a nucleolar grade 3 CCRCC and a stage 1b CDC.
Conclusions
Due to the rarity of RCT, there are no standard guidelines for their management. Hence, the prognosis is considered to be associated with the most aggressive component, possibly the tumour with the highest nucleolar grade and stage. The histogenesis of RCT remains debated, and increase in knowledge regarding this can help enable the development of targeted therapies for advanced or metastatic tumours.
Similar content being viewed by others
Background
Renal cell carcinoma (RCC) is a heterogeneous group of tumours representing ~ 2–4% of all adult cancers [1]. The risk factors for developing kidney tumours include smoking, obesity, high blood pressure, and exposure to certain toxic substances. The presence of multiple renal tumours is more frequently observed in people with hereditary conditions such as von Hippel-Lindau disease, Birt-Hogg-Dubé syndrome, hereditary papillary renal cell carcinomas (PRCC), hereditary leiomyomatosis, and tuberous sclerosis of Bourneville (TSB). Multifocality has also been observed in acquired cystic kidney disease and renal oncocytomatosis [2]. The incidence of sporadic multifocal renal tumours has been shown to vary between 4 and 20% at the time of diagnosis [3]. Tumours that are composed of a combination of different histological types in the same location within an organ are referred to as collision tumours, which have been reported to occur in several organs [4]. Here, we present three rare and sporadic cases of renal collision tumors (RCT), one involving clear cell renal cell carcinoma (CCRCC) and PRCC in a 64-year-old man, the second involving PRCC and angiomyolipoma (AML) in a 68-year-old woman, and the third involving CCRCC and duct collecting carcinoma (CDC) in a 59-year-old woman. All three cases were successfully treated by nephrectomy.
Case presentation
Case report 1
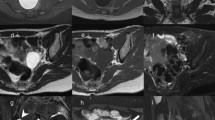
A 64-year-old man with a venous thromboembolic disease (heterozygous factor V mutation) underwent regular follow-ups. He was a uranium miner, had smoked for 50 years, and was an alcoholic. Thoraco-abdominopelvic computed tomography (CT) revealed a 100-mm lower-polar right renal cyst, classified as Bosniak IV with a 20-mm posterosuperior wall tissue nodule (Fig. 1A). A kidney biopsy showed a nucleolar grade 3 CCRCC combined with a nucleolar grade 2 type 1 PRCC.
The macroscopic, microscopic, and immunohistochemical features of the patient’s sample are described in Table 1 and Fig. 2.
Histologic features of collision renal tumour associating CCRCC and PRCC (hematoxylin and eosin, immunophenotype). Renal collision tumour combining two tumours: CCRCC «T1» and PRCC «T2» (A). Tumour T1 is composed of cystic and tubular pattern of clear cell carcinoma (B). Tumour T1 is constituted with atypical clear cell (C). Tumour T2 is arranged in papillary pattern (D). CK7 immunohistochemistry is negative in T1 (E) and positive in T2 (F) whereas CA-IX immunohistochemestry is positive in T1 (G) and negative in T2 (H)
The histological diagnosis of a RCT that combined a nucleolar grade 3 CCRCC measuring 110 mm (stage pT2; UICC 2017) and a nucleolar grade 2 type 1 PRCC measuring 16 mm (stage pT1a) was made, and an R0 excision was performed.
Twenty months into the clinical course, the patient is well and shows no evidence of tumour recurrence or metastatic lesions.
Case report 2
A 68-year-old woman underwent right partial nephrectomy for a right kidney tumour discovered during a check-up for pelvic trauma from a horse-riding accident. Magnetic resonance imaging revealed a heterogeneous 40 mm mid-renal mass with fat and tissue elements, which showed heterogeneous enhancement after gadolinium injection (Fig. 1B).
The macroscopic, microscopic, and immunohistochemical features of the patient’s sample are described in Table 1 and Fig. 3.
The histological diagnosis of a RCT with nucleolar grade 3 type 1 PRCC and AML was made. The tumour size and surgical margins could not be determined because the partial nephrectomy piece was fragmented.
The clinical course of the patient was unremarkable, and repeat CT did not show any recurrence after fourteen months of follow-up.
Case report 3
A 59-year-old woman was referred by her rheumatologist after a CT scan revealed a left kidney mass of 65 mm (Fig. 1C) during a chronic biological inflammatory syndrome check-up.
A kidney biopsy revealed a nucleolar grade 3 CCRCC. The patient underwent an enlarged left nephrectomy laparoscopically.
The macroscopic, microscopic, and immunohistochemical features of the patient’s sample are described in Table 1 and Fig. 4.
Histologic features of renal collision tumour associating CCRCC «T1» and CDC «T2» (hematoxylin and eosin, immunophenotype). Renal collision tumour combining two tumours: CCRCC «T1» and CDC «T2» (A). CD10 immunohistochemistry is positive in T1 and negative in T2 (B) whereas CD117 immunohistochemestry is negative in T1 and positive in T2 (C)
The histological diagnosis revealed a RCT consisting of a nucleolar grade 3 CCRCC measuring 28 mm (stage pT1a) and a CDC measuring 42 mm (stage pT1b).
At 12 months, the clinical evaluation was satisfactory and there was no recurrence.
Discussion and conclusions
The term ‘collision tumour’ refers to a phenomenon in which two or more distinct and unrelated tumour types are present at the same location, forming a single lesion. These tumours are usually limited and do not commonly undergo tumour changes [5]. However, it is challenging to distinguish between collision and composite tumours among the renal tumour cases cited in the literature. Similar to collision tumours, composite tumours consist of different tumours in a single lesion, but they do not have clear limits and usually undergo tumour changes. Synchronous tumours, however, comprise different tumour types in different locations within the same organ [6].
According to previous studies, collision tumours occur in many organs, including the lungs, digestive tract, and ovaries [4, 7, 8]. However, they rarely develop in the kidneys; most reported cases are unique ones [9]. Specifically, these tumours contain either two malignant components, as seen in Cases 1 and 3, or one malignant and one benign component, as seen in Case 2 [10, 11]. A review of 14 RCT cases with two previously published malignant components of renal origin along with our two cases (Cases 1 and 3) are presented in Table 2 [9, 11,12,13,14,15,16,17,18,19,20,21,22,23].
Studies have shown that RCC accounts for 2%–4% of adult malignancies and is predominant in men [1]. This was confirmed by the data collected in our study (Table 2), consisting of data from 10 male patients and 6 female patients aged 24–81 years (mean: 62 years). Moreover, the four main types of RCC (CCRCC, PRCC, chromophobe RCC, and CDC) are listed in Table 2. The most important associations were found in CCRCC and PRCC (5/16 cases), followed by chromophobe RCC and PRCC (3/16 cases). However, it was unfair to draw a conclusion regarding sex distribution and dominance of association due to the limited number of patients. Similar to classic renal tumours, collision tumours are usually discovered either incidentally or due to symptoms related to tumour expansion. Furthermore, smoking and exposure to industrial toxicants, as seen in Case 1, are risk factors associated with renal tumour development.
AML is considered the most common benign renal tumour, with a prevalence of 0.2%–0.6% of adult renal tumours and a female predominance. It is a mesenchymal tumour that belongs to the perivascular epithelioid cell group of tumours, which has been observed in nearly three-quarters of patients with certain hereditary factors such as TSB [2]. In Case 2, no hereditary or familial risk factors were identified. Similarly, renal AML in previous studies also appeared sporadically, but their association with other renal tumours was not well documented. Particularly, in a series of 36 collated cases of RCT associated with AML and renal cell tumours, Rafael et al. reported 25 sporadic cases and 11 TSB-related cases with mean ages of 59 and 53 years, respectively [24]. In this series, the association between AML and PRCC was found in only two of the 25 sporadic cases.
Regarding imaging appearance, collision tumours generally manifested atypical characteristics that offer limited information in the diagnostic process. In Case 1, CT was effective, consistent with the case reported by Goyal et al. [25]. The existence of possible collision tumours can be revealed on imaging when the capsule delimiting the two tumour components is well defined. However, when this border is not radiologically visible, as seen in Case 3 or in the cases reported by Gaeta et al. and McCroskey et al., radiological investigations may only show one mass [26, 27]. Therefore, it is essential to macroscopically sample the periphery of any renal mass to avoid misdiagnosis of any tumour association.
Recently, the interest in renal biopsy has re-emerged, especially in the management of small (less than or equal to 3 cm) renal solid masses [28]. Biopsy indications for small tumours, which has increasingly been discovered by imaging techniques, should be discussed during multidisciplinary conferences to establish a diagnosis that would guide the choice of management strategies. Although fine-needle biopsy can usually correctly classify three-fourths of classic renal tumours, renal biopsy may be more informative in cases of collision tumours if the biopsy fragments involve different tumour contents, as seen in Case 1 and in contrast to Case 3. So, the diagnosis frequency of collision renal masses obtained from percutaneous biopsy is not well documented. To our knowledge, the diagnosis of collision tumour made from percutaneous biopsies in case 1 is the first described in the English literature.
Despite the use of tumour biopsy, it can be challenging to analyse samples with two different co-existing histological types. A CCRCC and PRCC collision tumour (as seen in Case 1), in particular, must be distinguished morphologically from clear cell papillary renal cell carcinoma (CCPRCC), which is typically a low-grade renal carcinoma with histological aspects of both CCRCC and PRCC and was first described in 2006 [29]. CCPRCC displays a papillary, tubular, solid, or acinar architecture, with cells containing atypical nuclei that are polarised away from the basement membrane, creating characteristic sub-nuclear vacuoles such as the secretory endometrium [30]. Additionally, the axes of the papillae do not contain foamy macrophages or psammoma bodies, and the stroma occasionally exhibits mixed muscle fibre metaplasia. The tumour cells are positive for CA-IX and CK7 and negative for CD10 and P504S [30].
Notably, the prognosis of collision tumours is dependent on the nucleolar grade and RCC stage. In Case 3, the prognosis depended on both tumours as they were both high grade and Stage 1, whereas in Case 2, the prognosis of the tumour depended on PRCC since AML is a benign tumour. Furthermore, with the absence of tumour extension in the peri-renal adipose tissue in Case 2, complete tumour excision can be extrapolated. For collision tumours, it is essential to evaluate the relative share of the associated components, providing a histological type and stage for each. Thus, the prognosis is most likely related to the most aggressive component with the highest nucleolar grade and stage; however, there is no consensus on the clinical impact of CRT. Therefore, the treatment and follow-up of patients with CRT tumour have been based on standard guidelines for classic renal tumours [31].
The origin of the association between different tumour types in collision tumours is unclear. Therefore, different hypotheses have been proposed to explain its histogenesis. First, there may be two distinct cell lines proliferating simultaneously after a common oncogenic stimulus from the microenvironment, thereby causing two tumours with different phenotypes [12]. Second, the first tumour could alter the organ’s microenvironment, causing the second tumour to have a different phenotype [13]. Third, common precursor stem cells can differentiate into two different lineages and, therefore, cause two distinct tumours [14, 15]. Lastly, the appearance of two distinct tumours in the same anatomical site could have arisen accidentally after different oncogenic stimuli [32]. In Case 1, the two tumours, CCRCC and PRCC, had a common origin from the proximal tubule cells, thus pointing to the third hypothesis. In contrast, since AML originates from perivascular epithelioid cells different from PRCC, the histogenesis of Case 2 could be attributed to the second or fourth hypothesis. Moreover, since CCRCC originates from proximal tubule cells and CDC originates from distal tubule cells, the histogenesis of Case 3 would be similar to that of Case 2. Therefore, in-depth genomic studies on representative samples of collision tumours are fundamental for the elucidation of the somatic mutations responsible for these renal entities. Knowledge on the histogenesis mechanism of collision tumours can enable the development of targeted therapies for advanced or metastatic tumours.
In conclusion, collision tumours, which are composed of two distinct types of renal cell carcinoma, are rare and are usually discovered accidentally or due to symptoms related to tumour expansion. Histological diagnosis of biopsy specimens is challenging and requires exhaustive sampling of all associated tumour components. As the clinical impact of this entity has not been established due to its rarity, its management is based on standard guidelines for classic renal tumours. The histogenesis of such tumours is controversial, and its prognosis depends on the nucleolar grade and/or stage of the most aggressive component.
Availability of data and materials
Yes. The data can be found/requested from Limoges hospital archives. They can be consulted after making the request to the Research and Innovation Department (e-mail: abdeslam.bentaleb@chu-limoges.fr) specifying the title of case report, the authors and the ethics committee registration number (445–2021-101).
References
Cairns P. Renal cell carcinoma. Cancer Biomark. 2011;9:461–73.
Gaur S, Turkbey B, Choyke P. Hereditary Renal tumor syndromes: update on diagnosis and management. Semin Ultrasound CT MR. 2017;38:59–71.
Cifuentes-C L, Martínez CH, García-Perdomo HA, Cifuentes-C L, Martínez CH, García-Perdomo HA. Synchronous and multiple renal cell carcinoma, clear cell and papillary: an approach to clinically significant genetic abnormalities. Int Braz J Urol. 2020;46:287–93.
Bhattacharya A, Saha R, Biswas J, Biswas J, Ghosh B. Collision tumors in the gastrointestinal tract: a rare case series. Int Med Case Rep J. 2012;5:73–7.
Sung CT, Shetty A, Menias CO, Houshyar R, Chatterjee S, Lee TK, et al. Collision and composite tumors; radiologic and pathologic correlation. Abdom Radiol. 2017;42:2909–26.
Lall C, Houshyar R, Landman J, Verma S, Goyenechea M, Bhargava P, et al. Renal collision and composite tumors: imaging and pathophysiology. Urology. 2015;86:1159–64.
Suzuki S, Ohtsuka T, Hato T, Kamiyama I, Goto T, Kohno M, et al. Primary pulmonary collision tumor with three components in the underlying interstitial lung disease. Thorac Cancer. 2014;5:460–2.
Singh AKR, Singh M. Collision tumours of ovary: a very rare case series. J Clin Diagn Res JCDR. 2014;8:FD14–6.
Anani W, Amin M, Pantanowitz L, Parwani AV. A series of collision tumors in the genitourinary tract with a review of the literature. Pathol - Res Pract. 2014;210:217–23.
Arora K, Miller R, Mullick S, Shen S, Ayala AG, Ro JY. Renal collision tumor composed of oncocytoma and mucinous tubular and spindle cell carcinoma: case report of an unprecedented entity. Hum Pathol. 2018;71:60–4.
Zhang Z, Min J, Yu D, Shi H, Xie D. Renal collision tumour of papillary cell carcinoma and chromophobe cell carcinoma with sarcomatoid transformation: a case report and review of the literature. Can Urol Assoc J. 2014;8:E536–9.
Burch-Smith R, Tannir NM, Resetkova E, Tamboli P, Rao P. Collision tumor of the kidney composed of clear cell carcinoma and collecting duct carcinoma: report of a case with unusual morphology and clinical follow-up. Chin J Cancer. 2014;33:351–5.
Elaine T. Lam MD. Collision Renal Cell Papillary and Medullary Carcinoma in a 66-Year-Old Man. Cancer Network. 2013. https://www.cancernetwork.com/case-studies/collision-renal-cell-papillary-and-medullary-carcinoma-66-year-old-man. Accessed 19 May 2019.
Kawano N, Inayama Y, Nakaigawa N, Yao M, Ogawa T, Aoki I, et al. Composite distal nephron-derived renal cell carcinoma with chromophobe and collecting duct carcinomatous elements. Pathol Int. 2005;55:360–5.
Bartoš V. Collision tumor of the kidney composed of clear cell renal cell carcinoma and papillary renal cell carcinoma-a report of a unique case. J Res Med Dent Sci. 2018;6:3.
Matei D-V, Rocco B, Varela R, Verweij F, Scardino E, Renne G, et al. Synchronous collecting duct carcinoma and papillary renal cell carcinoma: a case report and review of the literature. Anticancer Res. 2005;25:579–86.
Cho NH, Kim S, Ha MJ, Kim HJ. Simultaneous heterogenotypic renal cell carcinoma: immunohistochemical and karyoptic analysis by comparative genomic hybridization. Urol Int. 2004;72:344–8.
Gong Y, Sun X, Iii GKH, Pins MR. Renal Cell Carcinoma, Chromophobe Type, With Collecting Duct Carcinoma and Sarcomatoid Components. :3.
Roehrl MHA, Selig MK, Nielsen GP, Dal Cin P, Oliva E. A renal cell carcinoma with components of both chromophobe and papillary carcinoma. Virchows Arch. 2007;450:93–101.
Moe A, Thyer I, Sinniah R, Hayne D. Open partial nephrectomy for a collision renal cell carcinoma in a transplant kidney: a case report. Urol Case Rep. 2020;33:101286.
Salazar-Mejía CE, Oyervides-Juárez VM, Wimer-Castillo BO, Vidal-Gutiérrez O, Garza-Guajardo R, Grande E. Collision tumor of the kidney composed of clear cell carcinoma and collecting duct carcinoma treated with cabozantinib and nivolumab. Curr Probl Cancer Case Rep. 2020;2:100039.
Lamprou S, Glykas I, Fragkoulis C, Theodoropoulou G, Koutsonikas G, Papadopoulos G. Collision kidney tumor with clear cell renal cell carcinoma and papillary type 1 renal cell carcinoma. A case report and review of the literature. Urol J. 2020. https://doi.org/10.1177/03915603211001673.
Compérat E. Tumeurs rares du rein. Cas no 8. Tumeurs de collision et tumeurs multiples. Ann Pathol. 2014;34(2):164–7.
Jimenez RE, Eble JN, Reuter VE, Epstein JI, Folpe AL, de Peralta-Venturina M, et al. Concurrent angiomyolipoma and renal cell neoplasia: a study of 36 cases. Mod Pathol. 2001;14:157–63.
Goyal R, Parwani AV, Gellert L, Hameed O, Giannico GA. A collision tumor of papillary renal cell carcinoma and oncocytoma: case report and literature review. Am J Clin Pathol. 2015;144:811–6.
Gaeta R, Tognetti A, Kauffmann EF, Pollina LE. Case report of a combined oncocytoma and type 1 papillary renal cell carcinoma: a rare entity. Pathol. 2019;111:37–40.
McCroskey Z, Sim SJ, Selzman AA, Ayala AG, Ro JY. Primary collision tumors of the kidney composed of oncocytoma and papillary renal cell carcinoma: a review. Ann Diagn Pathol. 2017;29:32–6.
Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9:44–55.
Tickoo SK, dePeralta-Venturina MN, Harik LR, Worcester HD, Salama ME, Young AN, et al. Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol. 2006;30:141–53.
Ross H, Martignoni G, Argani P. Renal cell carcinoma with clear cell and papillary features. Arch Pathol Lab Med. 2012;136:391–9.
Stempel K, Bencherki A, Tedehammar N, Sagen NE, Elzanaty S, Stempel K, et al. True collision renal tumour of oncocytoma and papillary renal cell carcinoma: case report and review of the literature. Arch Urol Res. 2020;4:080–4.
Aggarwal N, Amin RM, Chung D, Parwani AV. Tumor-to-tumor metastasis: case report of a pulmonary adenocarcinoma metastatic to a clear cell renal cell carcinoma. Pathol - Res Pract. 2012;208:50–2.
Acknowledgements
We would like to thank Alain Chaunavel (Clinical Research Associate) for logistical support.
Funding
No.
Author information
Authors and Affiliations
Contributions
I (VBM) certify that I and co-authors (FS, JPM, AD and FL) have read and contributed to the manuscript and all have agreed on its submission to this journal. VBM: microspopic analysis of renal specimens (biopsies and tumorectomies) and manuscript writing. FS: imaging analysis, carry out biopsies and manuscript review. AD: perform the surgery and manuscript review. JPM and FL: manuscript review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the several consents obtained from study participants was delivered on Monday, January 8, 2021 (number: 445-2021-101).
Consent for publication
The verbal consents of the three patients to the non-opposition regarding the use of their data (radiological or histological images and clinical details) were obtained prior to publication. This was approved by the ethics committee on Monday, January 8, 2021. In addition, patients were contacted to write the non-opposition to the use of their clinical data for research purposes.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Belle Mbou, V., Sanglier, F., Pestre-Munier, J. et al. Renal collision tumours: three additional case reports. BMC Urol 22, 113 (2022). https://doi.org/10.1186/s12894-022-01063-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-022-01063-y