Abstract
Background
An accurate prediction model could identify high-risk subjects of incident Overactive bladder (OAB) among the general population and enable early prevention which may save on the related medical costs. However, no efficient model has been developed for predicting incident OAB. In this study, we will develop a model for predicting the onset of OAB at 5-year in the general population setting.
Methods
Data will be obtained from the Nagahama Cohort Project, a longitudinal, general population cohort study. The baseline characteristics were measured between Nov 28, 2008 and Nov 28, 2010, and follow-up was performed every 5 years. From the total of 9,764 participants (male: 3,208, female: 6,556) at baseline, we will exclude participants who could not attend the follow-up assessment and those who were defined as having OAB at baseline. The outcome will be incident OAB defined using the Overactive Bladder Symptom Score (OABSS) at follow-up assessment. Baseline questionnaires (demographic, health behavior, comorbidities and OABSS) and blood test data will be included as predictors. We will develop a logistic regression model utilizing shrinkage methods (LASSO penalization method). Model performance will be evaluated by discrimination and calibration. Net benefit will be evaluated by decision curve analysis. We will perform an internal validation and a temporal validation of the model. We will develop a web-based application to visualize the prediction model and facilitate its use in clinical practice.
Discussion
This will be the first study to develop a model to predict the incidence of OAB.
Similar content being viewed by others
Background
Overactive bladder (OAB) is defined as “a symptom characterized by urinary urgency, with or without urgency incontinence, usually with urinary frequency and nocturia in the absence of infection or other obvious pathology” [1]. The prevalence of OAB is estimated from 10 to 20% and increases with age [2,3,4]. OAB might significantly decrease the HRQOL in patients [5] and increase the expenditure of medical cost [6]. The prevalence of OAB is increasing in an aging society and the negative impacts on HRQOL and medical cost are becoming even more serious.
Population-based prediction models would be helpful for population health planning and policy decision-making [7]. The same is expected for OAB prediction model because some good behaviors, such as healthy eating habit, keeping healthy weight, quitting smoking and pelvic floor muscle exercise, are efficient for keeping the bladder as healthy as possible [8]. If an accurate prediction model can be developed, high-risk subjects could be identified and encouraged to such good habits at an early stage, which might prevent incident OAB and save on the medical cost related to pharmacotherapy. If such a model could be made freely available to the general public online and encourage good habits for a healthy bladder, it could change user’s behavior to prevent incident OAB and impact on health care providers within clinical practice guidelines to inform decision making in the clinical setting. However, to our knowledge, no model has been developed to predict the new-onset of OAB in the literature. This could be due to lack of sufficient data to develop such a prediction model in terms of sample size, retrospective study design, and/or important predictors. We have recently reported longitudinal analyses of voiding dysfunction using a large prospective cohort data from the general population [9, 10]. These data can be used to develop an adequate model to predict new-onset OAB in the general population.
In this study, we will use a large prospective Japanese general population cohort to develop a model to predict the new-onset OAB at 5-year. We will develop a model consisting of only questionnaires and will compare the performance with another model including blood test. If the performance of the two models is deemed to be comparable, we will choose the model without blood testing, aiming to make the model more easily accessible, even by the general population. As the mechanism of incident OAB could be different between male and female due to factors such as the prostate gland, menopause and delivery, we will develop separate models for each sex. In addition, we will develop a web-based application to visualize the results interactively.
Methods/design
We will follow the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) checklist for developing and validating our prediction model [7].
Study design and source of data
We will use the Nagahama cohort [4, 9, 10], a prospective population-based cohort study in the Nagahama city, a Japanese rural city of 125,000 inhabitants. Recruitment was performed between Nov 28, 2008 and Nov 28, 2010, and the baseline characteristics were measured. Follow-up was performed every 5 years after baseline assessment, and the follow-up assessment was performed between July 28, 2013 and Feb 10, 2016. The cohort study was approved by the ethics committee of Kyoto University Graduate School of Medicine (no. G278) and by the Nagahama Municipal Review Board. Written informed consent was obtained from all participants.
Study population
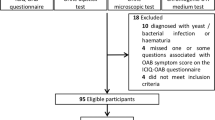
Participants were recruited from the general community residents of Nagahama city. Inclusion criteria were as follows: age 30–74 years; ability to independently participate in health examinations; no difficulties in communicating in Japanese; no serious diseases, symptoms, or other health issues; and voluntary participation. From the total 9764 participants (male: 3208, female: 6556) at baseline, we will exclude 1475 participants who did not attend the follow-up assessment because of death (n = 137), moving from Nagahama City (n = 279) or some other unknown reason (n = 1059). From the 8289 follow-up participant, we will exclude 912 OAB participant and 2 missing data of OAB at baseline, and 7375 participants (male: 2289, female: 5086) will be used in this analysis. The study flow chart is shown in Fig. 1.
Study outcome
The outcome will be new-onset OAB at 5-year follow-up assessment. We will use OABSS, a self-report measure assessing of urinary urgency validated by Homma et al. [11]. The questionnaire consists of the following items: (i) How many times do you typically urinate from waking in the morning until sleeping at night? (ii) How many times do you typically wake up to urinate from sleeping at night until waking in the morning? (iii) How often do you have a sudden desire to urinate, which is difficult to defer? (iv) How often do you leak urine because you cannot defer the sudden desire to urinate? OAB will be defined as a total OABSS score ≥ 3, with an urgency score (iii) ≥ 2 [11]. The number of new-onset OAB at follow-up assessment will be 224 for male and 290 for female.
Sample size calculation
We calculated the minimum sample size needed to build a prediction model using the criteria recommended by Riley et al. [12]. For these calculations it is required to provide an expected R2 value. As there has been no prediction model of incident OAB previously, we have set R2 = 0.10 as a conservative choice in this study. Based on the number of events in our dataset (224 male, 290 female) and the selected value for R2, we calculated the upper limit of the number of predictors to be 27 for the model for males and 35 for females.
Candidate predictor variables
Based on previous reports [13,14,15,16,17,18] and expert opinion, we will initially include in the mode the following candidate predictors variables which were measured at baseline; demographic variables (age, body mass index [BMI], delivery, menopause, smoking status, alcohol habit, walking habit), history of comorbidities (hypertension, hyperlipidemia, diabetes, ischemic heart disease, stroke, kidney disease, cancer, depression, sleep disturbance, obstructive sleep apnea [OSA], benign prostate disease and prostate cancer [PCa]), questionnaires specific to OAB (OABSS question 1, question 2, question 3 and question 4) and blood test (HbA1c, B-type natriuretic peptide [BNP], the estimated glomerular filtration rate [eGFR]) and prostate specific antigen [PSA]). Trained physicians and research assistants administered the standardized questionnaire in which participants provided clinical background information, such as lifestyle and medical history. Anthropometric and physiological measurements were taken by trained nurses.
Age will be treated as continuous value. BMI will be calculated as continuous values using height and weight data. Smoking status will be categorized as a dichotomous variable either current or none smoker. Alcohol habit will be categorized as a dichotomous variable either current or none drinker. Walking habit will be categorized as a dichotomous variable by the questionnaire: walking for ≥ 1 h or < 1 h. Delivery will be categorized as a dichotomous variable either experienced or not. History and medical comorbidities (menopause, hypertension, hyperlipidemia, diabetes, ischemic heart disease, stroke, kidney disease, cancer, depression, sleep disturbance, OSA, benign prostate disease, prostate cancer) will be categorized as a dichotomous variable by the questionnaire: yes or no. OABSS question 1, question 2, question 3 and question 4 will be treated as continuous variables. Blood samples (HbA1c, BNP, creatine, PSA) will be used as continuous values. eGFR will be calculated from serum creatinine levels using the following formula: 194 × serum creatinine−1.094 × age−0.287 (× 0.739 if female).
We will develop two models based on the sample size calculations as follows (Table 1a and b);
-
Model 1 including demographic questionnaires (age, BMI, delivery and menopause), health behavior questionnaires (smoking status, alcohol habit and walking habit) and comorbidities questionnaires (hypertension, hyperlipidemia, diabetes, ischemic heart disease, stroke, kidney disease, cancer, depression, sleep disturbance, OSA, prostate disease and prostate cancer) and questionnaires specific OAB (OABSS question 1, question 2, question 3 and question 4)
-
Model 2 consisting of Model 1 plus blood test (HbA1c, BNP, eGFR and PSA)
A total of 21 and 25 parameters of variables will be included in Model 1 and Model 2 for male, and 21 and 24 parameters will be included in Model 1 and Model 2 for female.
Data cleaning
We will create frequency tables for categorical variables and box plots for the continuous variables. We will identify values out of plausible range (i.e. values that are clearly erroneous), and we will classify them as missing data. We will exclude some categorical predictors with very small prevalence. Continuous variables will be standardized and categorical variables will be transformed into dummy variables.
Missing data
We will create 10 multiply imputed datasets using chained equations [19]. Each completed data set will be analyzed separately and the results will be combined by Rubin’s rules to account for imputation uncertainty [20].
Model development
Logistic regression model will be used to develop Model 1 and Model 2 to predict a binary outcome, new-onset OAB. To avoid overfitting of data, we will employ a shrinkage method (LASSO) [21]. To find the optimal hyperparameter (λ) of penalization, a tenfold cross-validation will be performed.
Model performance
We will evaluate the predictive accuracy of each model by R2 statistic. Model discrimination, i.e. the ability to classify the participants into high-risk or low-risk, will be evaluated using the C-statistic. Model calibration, agreement between observed outcomes and predictions, will be evaluated with calibration plots. To evaluate and compare the net benefit between models, decision curve analysis (DCA) will be performed [22].
Model validation
We will use internal validation and temporal validation to evaluate the model performance [23]. Internal validation will be performed via bootstrap procedure repeated 200 times to calculate optimism-corrected R2, c-statistics and calibration slope. Temporal validity will be assessed by splitting the sample into 3 sets according to the year of baseline assessment (i.e. 2008, 2009 and 2010). We will use the first 2 sets (2008 and 2009) as the training set, and the 2010 set as the testing set, to evaluate discrimination and calibration.
Statistical software
We will use R version 4.0.2 for our analyses. We will program a Shiny application in R to present the prediction results interactively.
Discussion
We have described the protocol for developing a prediction model for OAB. To our knowledge, this is the first model to predict new-onset OAB based on a large-scale prospective cohort in the general population setting. Our prediction models have a large sample size and will incorporate various predictive variables based on previous studies and expert opinions. Moreover, we will develop a user-friendly web-based application to visualize the results of the prediction model. This may be very useful not only to healthcare providers but also to the general population, in interpreting and understanding the results. If we can develop an accurate prediction model for OAB and make it widely available through a web app, we will be able to detect high risk populations and thus intervene at an early stage, which may improve individual HRQOL and decrease the societal health care expenditure.
There are some limitations in this study. First, there may be a selection bias in the sample because the study participants were recruited not by random sampling but on a voluntary basis. However, compared with the previous study using randomly sampled Japanese population [24], Nagahama cohort showed similar prevalence of OAB [4], which may indicate absence of potential selection bias. Second, we will not be able to perform an external validation using an independent cohort, therefore we will not evaluate the general applicability of the models. Future studies will be necessary to demonstrate the external validity of the models with another cohort data.
As a future perspective, prediction models of incident OAB will need to be externally validated and there should be an investigation of their impact in clinical practice [25]. Our models will be developed by general population data and predictors of Model1 will include only self-reported questionnaires. This study aims to develop a model that is easy to use in the general population setting, and thus easy validate externally.
Availability of data and materials
It is not possible to share research data publicly because individual privacy could be compromised.
Abbreviations
- BMI:
-
Body mass index
- BNP:
-
B-type natriuretic peptide
- DCA:
-
Decision curve analysis
- eGFR:
-
The estimated glomerular filtration rate
- HRQOL:
-
Health-related quality of life
- OAB:
-
Overactive bladder
- OABSS:
-
OAB symptom score
- OSA:
-
Obstructive sleep apnea
- PCa:
-
Prostate cancer
- PSA:
-
Prostate specific antigen
- TRIPOD:
-
Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis
References
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–78. https://doi.org/10.1016/S0090-4295(02)02243-4.
Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC Study. Eur Urol. 2006;50:1306–15.
Milsom I, Abrams P, Cardozo L, Roberts RG, Thüroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–6. https://doi.org/10.1046/j.1464-410X.2001.02228.x.
Funada S, Kawaguchi T, Terada N, Negoro H, Tabara Y, Kosugi S, et al. Cross-sectional epidemiological analysis of the Nagahama study for correlates of overactive bladder: genetic and environmental considerations. J Urol. 2018;199:774–8. https://doi.org/10.1016/j.juro.2017.09.146.
Vaughan CP, Johnson TM, Ala-Lipasti MA, Cartwright R, Tammela TLJ, Taari K, et al. The prevalence of clinically meaningful overactive bladder: bother and quality of life results from the population-based FINNO study. Eur Urol. 2011;59:629–36. https://doi.org/10.1016/j.eururo.2011.01.031.
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–8. https://doi.org/10.1111/j.1464-410X.2010.09993.x.
Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1-73.
Prevention of Bladder Control Problems (Urinary Incontinence) & Bladder Health | NIDDK. https://www.niddk.nih.gov/health-information/urologic-diseases/bladder-control-problems/prevention. Accessed 12 Jan 2021.
Funada S, Tabara Y, Negoro H, Akamatsu S, Yoshino T, Yoshimura K, et al. Longitudinal analysis of bidirectional relationships between nocturia and depressive symptoms: the Nagahama Study. J Urol. 2020;203:984–90. https://doi.org/10.1097/JU.0000000000000683.
Funada S, Tabara Y, Setoh K, Negoro H, Akamatsu S, Yoshino T, et al. Impact of nocturia on mortality: the Nagahama Study. J Urol. 2020;204:996–1002. https://doi.org/10.1097/JU.0000000000001138.
Homma Y, Yoshida M, Seki N, Yokoyama O, Kakizaki H, Gotoh M, et al. Symptom assessment tool for overactive bladder syndrome-overactive bladder symptom score. Urology. 2006;68:318–23.
Riley RD, Snell KIE, Ensor J, Burke DL, Harrell FE, Moons KGM, et al. Minimum sample size for developing a multivariable prediction model: PART II—binary and time-to-event outcomes. Stat Med. 2019;38:1276–96.
Dallosso HM, McGrother CW, Matthews RJ, Donaldson MMK. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int. 2003;92:69–77.
Link CL, Steers WD, Kusek JW, McKinlay JB. The association of adiposity and overactive bladder appears to differ by gender: results from the Boston area community health survey. J Urol. 2011;185:955–63. https://doi.org/10.1016/j.juro.2010.10.048.
Ikeda Y, Nakagawa H, Ohmori-Matsuda K, Hozawa A, Masamune Y, Nishino Y, et al. Risk factors for overactive bladder in the elderly population: a community-based study with face-to-face interview. Int J Urol. 2011;18:212–8. https://doi.org/10.1111/j.1442-2042.2010.02696.x.
Ohgaki K, Horiuchi K, Kondo Y. Association between metabolic syndrome and male overactive bladder in a Japanese population based on three different sets of criteria for metabolic syndrome and the Overactive Bladder Symptom Score. Urology. 2012;79:1372–8. https://doi.org/10.1016/j.urology.2012.03.006.
Hirayama A, Torimoto K, Mastusita C, Okamoto N, Morikawa M, Tanaka N, et al. Risk factors for new-onset overactive bladder in older subjects: results of the Fujiwara-kyo study. Urology. 2012;80:71–6. https://doi.org/10.1016/j.urology.2012.04.019.
Kurita N, Yamazaki S, Fukumori N, Otoshi K, Otani K, Sekiguchi M, et al. Overactive bladder symptom severity is associated with falls in community-dwelling adults: LOHAS study. BMJ Open. 2013;3:e002413.
van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Rubin DB, Schenker N. Multiple imputation in health-are databases: an overview and some applications. Stat Med. 1991;10:585–98. https://doi.org/10.1002/sim.4780100410.
Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B. 1996;58:267–88.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak. 2006;26:565–74. https://doi.org/10.1177/0272989X06295361.
Steyerberg EW. Clinical prediction models. New York: Springer New York; 2009. https://doi.org/10.1007/978-0-387-77244-8.
Homma Y, Yamaguchi O, Hayashi K. An epidemiological survey of overactive bladder symptoms in Japan. BJU Int. 2005;96:1314–8. https://doi.org/10.1111/j.1464-410X.2005.05835.x.
Steyerberg EW, Moons KGM, van der Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Med. 2013;10:e1001381.
Acknowledgements
We are extremely grateful to the Nagahama City Office and non-profit organization Zeroji Club for their help in performing the Nagahama Study. The Nagahama Study group executive committee is composed of the following individuals: Yasuharu Tabara, Takahisa Kawaguchi, Kazuya Setoh, Yoshimitsu Takahashi, Shinji Kosugi, Takeo Nakayama, and Fumihiko Matsuda from the Center for Genomic Medicine, Kyoto University Graduate School of Medicine (Ya.T, T.K., K.S., F.M.); and the Department of Health Informatics (Yo.T, T.N.) and, Department of Medical Ethics and Medical Genetics (S.K.), Kyoto University School of Public Health.
Funding
The Nagahama study was supported by a university grant, the Center of Innovation Program, the Global University Project, and a Grant-in-Aid for Scientific Research (25293141, 26670313, 26293198, 17H04182, 17H04126, 17H04123, 18K18450) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Practical Research Project for Rare/Intractable Diseases (ek0109070, ek0109070, ek0109196, ek0109348), the Comprehensive Research on Aging and Health Science Research Grants for Dementia R&D (dk0207006, dk0207027), the Program for an Integrated Database of Clinical and Genomic Information (kk0205008), the Practical Research Project for Lifestyle-related Diseases including Cardiovascular Diseases and Diabetes Mellitus (ek0210066, ek0210096, ek0210116), and the Research Program for Health Behavior Modification by Utilizing IoT (le0110005), from Japan Agency for Medical Research and Development (AMED); the Takeda Medical Research Foundation; the Mitsubishi Foundation; the Daiwa Securities Health Foundation; and the Sumitomo Foundation. This study protocol was supported by a research grant from the KDDI Foundation. These funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and conduct of this clinical trial. OO is the principal investigator of this study protocol. SF, YL, TY, SA, TAF, OE and OO designed the overall framework of the trial and KS, YT, HN, KY and FM helped with implementation. SF, YL, TAF and OE drafted the manuscript, and all co-authors read it and provided critical comments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of Kyoto University Graduate School of Medicine (no. G278) and by the Nagahama Municipal Review Board. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
SF has a research grant from, the Ministry of Education, Culture, Sports, Science and Technology of Japan, JSPS KAKENHI Grant Number JP 20K18964. SA has a research grants from Astellas, grants from Astra Zeneca, grants from Tosoh. TAF reports grants and personal fees from Mitsubishi-Tanabe, personal fees from MSD, personal fees from Shionogi, outside the submitted work; In addition, TAF has a patent 2018-177688 concerning smartphone CBT apps pending, and intellectual properties for Kokoro-app licensed to Mitsubishi-Tanabe. OE was supported by the Swiss National Science Foundation (Ambizione grant number 180083). All of the other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Funada, S., Luo, Y., Yoshioka, T. et al. Protocol for development and validation of a prediction model for 5-year risk of incident overactive bladder in the general population: the Nagahama study. BMC Urol 21, 78 (2021). https://doi.org/10.1186/s12894-021-00848-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-021-00848-x