Abstract
Background
Our purpose was to study the relationship of the 3 different types of endoscopic calcifications of the renal papilla (Randall’s plaque, intratubular calcification, papillary crater) with the type of stone and urine analysis.
Methods
This prospective study examined 41 patients (age range: 18 to 80 years) who received retrograde intrarenal surgery (RIRS) for renal lithiasis (mean stone size: 15.3 ± 7.2 mm). The renal papilla injuries were endoscopically classified as Randall’s plaque, intratubular calcification, or papillary crater. Calculi were classified as uric acid, calcium oxalate monohydrate (COM; papillary and cavity), calcium oxalate dihydrate (COD), or calcium phosphate (CP). A 24 h urine analysis of calcium, oxalate, citrate, phosphate, and pH was performed in all patients. The relationship of each type of papillary injury with type of stone and urine chemistry was determined. Fisher’s exact test and Student’s t-test were used to determine the significance of relationships, and a p value below 0.05 was considered significant.
Results
The most common injury was tubular calcification (78%), followed by Randall’s plaque (58%), and papillary crater (39%). There was no significant relationship of Randall’s plaque with type of stone. However, endoscopic intratubular calcification (p = 0.025) and papillary crater (p = 0.041) were more common in patients with COD and CP stones. There were also significant relationships of papillary crater with hypercalciuria (p = 0.036) and hyperoxaluria (p = 0.024), and of Randall’s plaque with hypocitraturia (p = 0.005).
Conclusions
There are certain specific relationships between the different types of papillary calcifications that were endoscopically detected with stone chemistry and urine analysis. COD and CP stones were associated with endoscopic tubular calcifications and papillary craters. Hypercalciuria was associated with tubular calcification, and hypocitraturia was associated with Randall’s plaque.
Similar content being viewed by others
Background
In 1937 Alexander Randall first established a relationship between a non-inflammatory subepithelial calcification and the onset of kidney stone formation [1]. Although many subsequent studies have examined the mechanisms and causes of Randall’s plaques, the conclusions of many of these studies still require verification [2].
Papillary calculi can initiate in the papillary tissue or inside the Bellini ducts, and there are therefore two different types of stone formation. First, calculi may initiate in papillary tissue following oxidative stress, exposure to cytotoxic substances, or other factors that injure collagen-rich areas near the loop of Henle and the cubic epithelium, which covers the papilla. Because the interstitial fluid has a pH of 7.4, and is therefore always supersaturated with calcium phosphate, cellular detritus or oxidized cellular collagen can act as a heterogeneous nucleant, leading to the deposit of hydroxyapatite (HAP). If this deposit grows enough to break through the epithelium that covers the papilla, it can contact the urine. Because the urine is always supersaturated with calcium oxalate, a calcium oxalate monohydrate (COM) calculus will form when there is a deficiency of crystallization inhibitors and/or an alteration of the immune system. Stones can also form when there is intratubular injury of the Bellini ducts and urine supersaturation, in which there are large amounts of calcium oxalate dihydrate (COD) and/or HAP crystals. In this case, obstruction of the collecting tubules and deposits that form in the most distal part of the tubule, upon contacting the urine, generate the renal calculi [3,4,5].
Obviously, not all kidney stones develop by processes that involve the renal papillae. Thus, the presence of morpho-anatomical factors, such as cavities that have low urodynamic efficacy and anatomical malformations, can lead to urine stasis, the accumulation of stagnant urine, and stone formation, as also occurs in the urinary bladder. The renal papillae have no role in the formation of these stones [5,6,7].
Because of advances in urological technologies and endoscopy, and the increasing comfort and dexterity of the surgeons in performing endoscopic procedures, retrograde intrarenal surgery (RIRS) has become a common method for treatment of renal stones. These techniques of renal exploration allow better treatment of renal stones and more thorough examination of the renal papillae [8, 9]. Although endoscopic renal surgery can detect a wide range of injuries to the renal papilla surface, the relationships of these different injuries with the etiopathogenic mechanisms of renal stone formation are unclear [10].
The purpose of this paper is to study the relationship between injuries detected endoscopically of the renal papilla detected by endoscopy with the type of renal calculus and 24 h urine analysis.
Methods
Forty-one consecutive patients with renal lithiasis and indications for RIRS were recruited prospectively from our institution. All patients provided informed consent for participation in the study, and the study was approved by the ethics committee of our hospital (No. 3105/15P, Comité de Ética de la Investigación de las Islas Baleares- CEI-IB). All research was performed in accordance with relevant guidelines/regulations.
We measured stone size using computed tomography prior to surgery. Systematic endoscopic examination of the renal papilla was performed using a flexible digital ureteroscope (Olympus URF-V and Karl Storz Flex Xc). The procedure started with examination of the upper calyx, ended in the lower calyx, and photographs were taken in the area of the renal papillae. The 3 types of injury in the papilla were defined as previously described [11]:
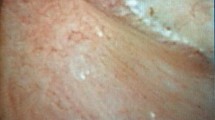
Randall’s plaque (Fig. 1): White deposits of intrarenal HAP calcifications, near the epithelium of the papilla, with irregular shape.
Intratubular calcification or Randall’s plug (Fig. 2): Yellow punctate deposit on the surface of the renal papilla or at the end of a dilated terminal collection tubule.
Papillary crater (Fig. 3): Slit-shaped injury or crater in the surface of a renal papilla.
After laser lithotripsy, some stone fragments were extracted to determine calculus type. In particular, electron microscopy and infrared spectroscopy were used to classify stones into 1 of 4 groups: calcium oxalate monohydrate (COM; papillary and cavity), calcium oxalate dihydrate (COD), uric acid, and calcium phosphate (HAP and mixed stones) [12, 13]. Struvite and brushite stones were not considered because their mechanisms of formation are completely different.
At 30 to 60 days after surgery, 24 h urinary chemistry was analyzed in each patient. The results were used to classify patients as having hypercalciuria (> 220 mg/24 h or > 17 mg/dL), hyperoxaluria (> 40 mg/24 h or > 3 mg/dL), hypocitraturia (< 350 mg/24 h or < 23 mg/dL), or hyperphosphaturia (> 1100 mg/24 h or > 100 mg/dL), as previously described [12,13,14].
The relationships of each endoscopic papillary injury with type of stone, urinary biochemical parameters, and urinary pH were determined. Each value is reported as mean ± standard deviation. Fisher’s exact test was used for the analysis of qualitative variables, and the Student’s t-test for the comparison of means. A p value below 0.05 was considered significant.
Results
The average patient age was 48 years (±13), and 54% of the patients were male. A total of 58% of the patients had Randall’s plaque, 78% had intratubular calcification, and 39% had a papillary crater. Thus, 54% of the patients had 2 or more different types of renal injury, and 46% only had one kind of renal injury.
The stone mean size (i.e., largest diameter) was 15.4 mm (±7.4). Urinary biochemistry indicated the mean pH was 6.0 (±0.79), mean 24 h urine volume was 1863 mL (±901), mean calcium level was 218 mg/24 h (±127), mean phosphate level was 835 mg/24 h (±312), mean oxalate level was 27 mg/24 h (±12), and mean citrate level was 398 mg/24 h (±300).
There was no significant relationship of Randall’s plaque with the type of stone (p = 0,39) (Table 1). However, there were significant relationships of endoscopic intratubular calcification (p = 0.025) and papillary crater (p = 0.041) with type of stone, in that COD and calcium phosphate stones were more common in both groups (Tables 2 and 3).
There were also significant relationships between papillary crater and hypercalciuria (p = 0.036) and hyperoxaluria (p = 0.024), and between Randall’s plaque and hypocitraturia (p = 0.005). None of the other relationships were statistically significant.
Discussion
Our results suggest that the type of papillary injury identified by endoscopy is associated with the type of stone and with certain disorders in urinary analysis. This suggests that different mechanisms are responsible for these different endoscopic papillary injuries.
Coe et al. [15] hypothesized that the direct contact of a plug blocking Bellini’s duct in an environment with hypercalciuric urine favors the formation of HAP and calcium oxalate stones, although this relationship has not been demonstrated previously. Our results are consistent with this hypothesis. In particular, we observed that intratubular calcification and papillary crater each had significant associations with the type of lithiasis, in that they were more prevalent in patients with COD and CP stones. Moreover, we found that Randall’s Plaque was mainly associated with COM calculi.
We also found that intratubular calcification was significantly associated with urinary calcium concentration. This result confirms that Bellini’s duct must be exposed to supersaturated urine for the formation of HAP stones (pH > 6) and to hypercalciuria for the formation of an intratubular obstruction. When this deposit reaches the end of Bellini’s duct and contacts the urine, it can induce the development of COD or COD+HAP calculi.
On the other hand, Randall’s plaque was mainly associated with COM calculi that developed in renal cavities. It is important to consider that typical calculi of papillary COM are generated on Randall’s plaque, which originates from internal lesions of intrapapillar tissue [6]. These are small stones that are usually expelled spontaneously. Consequently, this type of calculus does not require surgery. However, all the stones examined in the present study required RIRS for elimination because they were larger, developed on the most distal part of Bellini’s ducts, and had fast growth due to the existence of hypercalciuria and sometimes high urinary pH. Therefore, we expected that none of the included patients had typical COM papillary calculi. We did observe the presence of Randall’s plaques, which in principle are related to the formation of COM stones. However, recent studies showed that not all of Randall’s plaques generate papillary COM calculi [6].
In fact, Randall’s plaque seems to occur without symptoms in most individuals, and stone formation only occurs when there are large numbers of these plaques [6]. It is thus possible that the small number of Randall’s plaque lesions and their total area in these patients were insufficient to lead to the development of papillary COM stones. We did not evaluate the percentage of surface affected by Randall’s plaque. However, other studies reported that the surface affected by Randall’s plaque was 7.4% in those who developed papillary calculi, but only 0.5% in those who did not develop calculi [16,17,18,19,20,21,22].
We found that Randall’s plaque was significantly associated with hypocitraturia (p = 0.005) and that urinary calcium concentration was significantly lower than those detected in the presence of intratubular calcification and / or renal crater. These data are consistent with the current understanding that a deficiency of stone inhibitors and normocalciuria can lead to the development of COM calculi [6]. Thus, citrate inhibits the crystallization of oxalate and calcium phosphate, preventing the formation of the lithiasic core and posterior growth. Interestingly, regardless of the type of injury, the presence of uric acid stones is more likely when the urinary pH is below 5.5, and the presence of HAP stones is more likely when the urinary pH is above 6.0 [23,24,25,26,27,28,29].
There were some limitations in our study. First, we only described the experience of 41 patients at a single center. Second, the identification of papillary injury was somewhat subjective, even though we used previously described criteria to define the different types of injuries. Third, we did not consider the severity of papillary injuries, and only considered the presence or absence of a lesion. Another possible limitation is that there may have been some overlap in the different types of lesions if they were at different stages of disease progression. For example, we found an association of intratubular calcification with papillary crater. This can occur if lithiasis begins in an intratubular calcification, but detachment of the calculus from the renal papilla leads to the formation of a papillary crater (Fig. 4). The last limitation of the study is that the urine analysis was done at the 30–60 day after surgery and this could make changes in the urine chemistry.
Our study identified several significant relationships of endoscopic papillary injuries with the type of stone and urine chemistry. The characterization of a papillary endoscopic injury may help a urologist to better understand the underlying mechanism of stone formation in individual patients. Further studies with larger numbers of patients are needed to confirm this hypothesis.
Conclusions
We identified certain specific relationships between different endoscopic types of papillary calcifications with stone and urine chemistry. In particular, COD and CP stones are associated with endoscopic tubular calcifications and papillary craters; hypercalciuria is associated with endoscopic tubular calcification; and COM calculi, hypocitraturia, and normocalciuria are associated with Randall’s plaque.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- UA:
-
Uric Acid
- COM:
-
Calcium oxalate monohydrate
- COD:
-
Calcium oxalate dihydrate
- CP:
-
Calcium phosphate
References
Randall A. The origin and growth of renal calculi. Ann Surg. 1937;105(6):1009–27.
Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 2010;25(5):831–41.
Evan AP, Worcester EM, Coe FL, Williams J, Lingeman JE. Mechanisms of human kidney stone formation. Urolithiasis. 2015;43(S1):19–32.
Coe FL, Evan AP, Worcester EM, Lingeman JE. Three pathways for human kidney stone formation. Urol Res. 2010 Jun 22;38(3):147–60.
Bird VY, Khan SR. How do stones form? Is unification of theories on stone formation possible? Arch Esp Urol. 2017;70(1):12–27.
Grases F, Costa-Bauza A, Ramis M, Montesinos V, Conte A. Simple classification of renal calculi closely related to their micromorphology and etiology. Clin Chim Acta. 2002;322:29–36.
Gambaro G, Trinchieri A. Recent advances in managing and understanding nephrolithiasis/nephrocalcinosis. F1000Research. 2016;5:695.
Reis Santos JM. Ureteroscopy from the recent past to the near future. Urolithiasis. 2018;46:31–7.
Rassweiler J, Fiedler M, Charalampogiannis N, et al. Robot-assisted flexible ureteroscopy: an update. Urolithiasis. 2018;46:69–77.
Almeras C, Daudon M, Ploussard G, Gautier JR, Traxer O, Meria P. Endoscopic description of renal papillary abnormalities in stone disease by flexible ureteroscopy: a proposed classification of severity and type. World J Urol. 2016 Nov;34(11):1575–82.
Borofsky MS, Paonessa JE, Evan AP, Williams JC, Coe FL, Worcester EM, et al. A proposed grading system to standardize the description of renal papillary appearance at the time of endoscopy in patients with nephrolithiasis. J Endourol. 2016;30(1):122–7.
Norman RW, Bath SS, Robertson WG, Peacock M. When should patients with symptomatic urinary stone disease be evaluated metabolically? J Urol. 1984;132(6):1137–9.
Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med. 1995 Jan;98(1):50–9.
Tiselius HG. An improved method for the routine biochemical evaluation of patients with recurrent calcium oxalate stone disease. Clin Chim Acta. 1982;122(3):409–18.
Coe FL, Worcester EM, Evan AP. Idiopathic hypercalciuria and formation of calcium renal stones. Nat Rev Nephrol. 2016;12(9):519–33.
Aggarwal KP, Narula S, Kakkar M, Tandon C. Nephrolithiasis: Molecular Mechanism of Renal Stone Formation and the Critical Role Played by Modulators. Biomed Res Int. 2013;2013:1–21.
Chung H. The role of Randall plaques on kidney stone formation. Transl Androl Urol. 2014;3(3):251–4.
Haggitt RC, Pitcock JA. Renal medullary calcifications: a light and electron microscopic study. J Urol. 1971 Sep;106(3):342–7.
Williams JC, Matlaga BR, Kim SC, Jackson ME, Sommer AJ, McAteer JA, et al. Calcium oxalate calculi found attached to the renal papilla: preliminary evidence for early mechanisms in stone formation. J Endourol. 2006;20(11):885–90.
Evan A, Lingeman J, Coe FL, Worcester E. Randall’s plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006;69(8):1313–8.
Daudon M, Bazin D, Letavernier E. Randall’s plaque as the origin of calcium oxalate kidney stones. Urolithiasis. 2015;43(S1):5–11.
Kuo RL, Lingeman JE, Evan AP, Paterson RF, Parks JH, Bledsoe SB, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int. 2003;64(6):2150–4.
Low RK, Stoller ML. Endoscopic mapping of renal papillae for Randall’s plaques in patients with urinary stone disease. J Urol. 1997;158(6):2062–4.
Wang X, Krambeck AE, Williams JC, Tang X, Rule AD, Zhao F, et al. Distinguishing characteristics of idiopathic calcium oxalate kidney stone formers with low amounts of Randall’s plaque. Clin J Am Soc Nephrol. 2014 Oct 7;9(10):1757–63.
Strohmaier WL, et al. Papillary calcifications: a new prognostic factor in idiopathic calcium oxalate urolithiasis. Urolithiasis. 2013;41:475–9.
Daudon M, Dessombz A, Frochot V, Letavernier E, Haymann J-P, Jungers P, et al. Comprehensive morpho-constitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. Int J Surg. 2016;36(Part D):624–32.
Hesse A, Kruse R, Geilenkeuser W-J, Schmidt M. Quality control in urinary stone analysis: results of 44 ring trials (1980–2001). Clin Chem Lab Med. 2005;43(3):298–303.
Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111(5):607–16.
Evan AP, Lingeman JE, Worcester EM, Sommer AJ, Phillips CL, Williams JC, et al. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxy apatite, brushite, or calcium oxalate stones. Anat Rec (Hoboken). 2014;297(4):731–48.
Acknowledgements
IUNICS lab. Universitat de les Illes Balears.
Funding
There was no funding for the study.
Author information
Authors and Affiliations
Contributions
SA, XA: Project development, Data Collection, Data analysis, Manuscript writing. GF, F.: Project development, Manuscript Writing. BQ, JL.: Data Collection. GG, J: Data Collection. PA, E: Project development, Data Collection, Data analysis, Manuscript writing. All Authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients provided written informed consent for participation in the study, and the study was approved by the ethics committee of our hospital (No. 3105/15P, Comité de Ética de la Investigación de las Islas Baleares- CEI-IB). All research was performed in accordance with relevant guidelines/regulations.
Consent for publication
Not applicable.
Competing interests
All the authors have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sabaté Arroyo, X., Grases Freixedas, F., Bauzà Quetglas, J.L. et al. Relationship of endoscopic lesions of the renal papilla with type of renal stone and 24 h urine analysis. BMC Urol 20, 46 (2020). https://doi.org/10.1186/s12894-020-00615-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-020-00615-4