Abstract
Background
Extracranial carotid artery aneurysm (ECAA) is a rare peripheral arterial disease. The main treatment strategies include conservative treatment, open surgery, endovascular treatment, and hybrid techniques, and there is no expert consensus or guidelines, with only a few case reports.
Method
This article reviewed 10 cases diagnosed with "extracranial carotid artery aneurysm" and received invasive treatment from January 2013 to July 2023 in our medical center.
Results
There were 10 patients with ECAA admitted to our center, including seven cases of true aneurysms, two cases of pseudoaneurysms, and one case of dissecting aneurysm. There were 3 females and 7 males aged between 24–61 years. Based on the characteristics of ECAA, we designed the individualized procedure including open surgery, endovascular treatment, and hybrid treatment. Procedures were technically successful for all patients, and none of them had any adverse events during the follow-up period except for one patient who developed cerebral hemorrhage on the third postoperative day and recovered after cerebral puncture and drainage.
Conclusion
The current invasive treatments for ECAA mainly include open surgery, endovascular treatment, and hybrid treatment, and they all appear to be safe and effective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Extracranial carotid artery aneurysm (ECAA) is an ultra-rare form of the peripheral aneurysm, with only 1,000 cases reported in the world literature so far [1]. Depending on the morphology, aneurysms can be classified as true aneurysms, pseudoaneurysms, and dissecting aneurysms; true aneurysms are most often caused by atherosclerosis, trauma is often the cause of pseudoaneurysms and dissecting aneurysms often occur at the site of the most pronounced changes in blood pressure. Attigah et al. classified carotid aneurysms into 5 types based on anatomical location. Type I: isolated and short aneurysms of the internal carotid artery (ICA) above the carotid bulb. Type II: long aneurysms of the ICA ranging from the carotid bulb to Blaisdell’s line. Type III: aneurysms of the proximal part of the ICA and the carotid bifurcation. Type IV: aneurysms involving the common carotid artery (CCA) and the ICA as with the Type III, extending distally and proximally. type V: isolated aneurysm of the CCA [2].
Treatment of ECAA includes open surgery, endovascular treatment, hybrid operation, and conservative treatment with antiplatelet and anticoagulant agents, however, there are no treatment guidelines or expert consensus to date. This article summarised the case characteristics, anatomical features, and treatment measures of 10 cases of ECAA that have undergone surgical treatment in our center over the past 10 years.
Method
In this article, we reviewed 10 cases diagnosed of "extracranial carotid artery aneurysm" and treated with invasive therapy from January 2013 to July 2023 in our center. The data were obtained from electronic medical records. It was diagnosed in all patients by digital subtraction angiogram (DSA), computed tomography angiography (CTA) or duplex scan. ECAA was defined as expansion of vessel diameter to 1.5 times the expected vessel diameter. The aneurysm was identified as a true aneurysm, pseudoaneurysm, and dissecting aneurysm based on preoperative CTA or intraoperative DSA characteristics. Like other sites of aneurysms, true aneurysms could be seen with aneurysmal dilatation of the arterial wall, pseudoaneurysms could be seen with contrast leakage of the vascular wall on angiography, and dissecting aneurysms could be seen an added pseudovascular lumen formed by the arterial media. Patients without any accompanying manifestations of aneurysm (e.g., pulsatile mass, peripheral organ or tissue compression) and associated neurologic symptoms were considered asymptomatic [3]. We conducted invasive treatments for both symptomatic and asymptomatic patients. The follow-up was conducted through outpatient follow-up record review and telephone inquiries.
Result
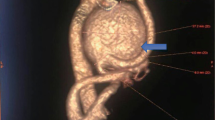
Over the past 10 years, ten patients with ECCA were treated at our center including seven cases of true aneurysms, two cases of pseudoaneurysms and one case of dissecting aneurysm. The characteristics of the cases are shown in Table 1, consisting of three females and seven males, in the age range of 24–61 years old. The right carotid artery was involved in 4 cases and the left carotid artery in 6 cases. Regarding the initial symptoms, there were three cases of pulsatile mass in the neck, three cases of dizziness, two of which were accompanied by headache, two cases of numbness and weakness of the contralateral limbs, and one case of numbness of the left eyelid probably due to compression of the external carotid artery by the mass. Four of them had previous cerebral infarction, four suffered from hypertension, three from peripheral arterial disease, and one from coronary heart disease. Two patients had a smoking history. According to the classification of Attigah et al., eight cases were type I, remaining two were type II and type IV respectively. The preoperative CTA or DSA, and intraoperative DSA of the cases were shown in Figs. 1 and 2. Among the patients with type I, two patients with pseudoaneurysms, one patient with true aneurysm and one patient with dissecting aneurysm underwent endovascular treatment; three patients with true aneurysm underwent open surgery, with end-to-end anastomosis; and one patient underwent individualized hybrid procedures (Fig. 1). The type II patient underwent hybrid procedure and the type V patient underwent endovascular treatment. Surgical procedures were successful in all patients (Fig. 2). Open surgery and hybrid surgery patients received preoperative mono-antiplatelet therapy medication, while endovascular therapy patients received preoperative dual antiplatelet therapy, for at least 3 days.
The preoperative image assessment and intraoperative digital subtraction angiogram (DAS) of type I cases. Image (a), (b), and (c) show the preoperative assessment and intraoperative DAS of case 4; (d), (e), and (f) show case 10; (g), (h), and (i) show case 3; (j) shows case 9. The CTA (a), DSA (d), (g), and (j) show the type I dissecting aneurysm and type I aneurysms preoperatively; DSA (b), (c), (e), and (f) show the ECAA treated with endovascular treatment has disappeared and target artery patency without endoleak. DSA (h) and (i) show the ECAA treated with hybrid procedure. The yellow arrows point out the dissecting aneurysms. Red arrows point out stents
The preoperative image assessment and intraoperative digital subtraction angiogram (DAS) of type II and V cases. Image (a), (b), and (c) show the preoperative assessment and intraoperative DAS of case 7; (d), (e), and (f) show case 2. The DSA (a) and (d) show the type II and V aneurysms preoperatively; DSA (b) and (c) show the ECAA treated with hybrid procedure. DSA (e) and (f) show the ECAA treated with endovascular treatment has disappeared and target artery patency without endoleak. Red arrows point out stents
The patients were followed up for 7–72 months. It was recommended that patients be followed up by ultrasound at 3 months, 6 months, 12 months postoperatively and every following year, with CTA when ultrasound results indicated a stenosis > 50% or when relevant symptoms were developed. All patients without lost follow-up received dual antiplatelet therapy for at least 3 months postoperatively and long-term mono-antiplatelet therapy thereafter. The first case occurred right frontal lobe cerebral hemorrhage on the third postoperative day, which was recovered after cerebral puncture and drainage. The nine remaining patients did not have any adverse events during the perioperative period, and had no new ipsilateral cerebral infarct during the follow-up period, with patency of blood flow through the treated blood vessels.
Discussion
ECAA is rarely observed, accounting for less than 1% of all aneurysms, and has been published mostly as a case report [4]. Most of the ECAA is mostly located at the carotid bifurcation or distal to the ICA, and its aetiology includes atherosclerosis, infection, fibromuscular dysplasia, connective tissue disease, trauma, previous surgery, radiation, or spontaneous entrapment [5, 6]. Initial symptom was most often reported as cerebral thromboembolism and partial compression, while rupture of the ECAA was suspected to be underreported [7, 8]. The anatomical location and characterization of the ECAA in our study cohort followed the same trend.
ECAA has typically been diagnosed by clinical symptoms and imaging examinations. A pulsatile neck mass could be found in up to 93% of patients, and neurologic symptoms were reported in about 50% of patients in previous studies [2, 9, 10]. And with the frequent use of imaging devices, asymptomatic ECAAs were increasingly being diagnosed. Grant T et al. reported that 49% of ECAAs in their case series were found incidentally [6]. Ultrasound is a convenient and reliable tool for the distinction of a mass as a solid tumor or a vascular disease, while CTA could be used to inform the surgical procedure [11]. In our center, the diagnosis was based on the symptoms of the patient and the ultrasound results, combined with the CTA results to provide additional information for procedures, and the follow-up was mainly based on the ultrasound results.
Invasive treatment has been advocated for any ECAA, whether symptomatic or asymptomatic, due to the high mortality rate of non-invasive cases [12]. In 1808, Cooper documented the first successful ECAA procedure, the proximal ligation; although the method was reported to have a high stroke rate of 25% and a high mortality rate of 20%, it was used until the early 1950s [13]. Resection of the aneurysm with target artery reconstruction was first successfully performed in 1956, and open repair reduced postoperative mortality and stroke rates, making carotid artery reconstruction the gold standard treatment [14, 15]. In 2009 Attigah et al. typed ECAA based on the anatomical location of the aneurysm and its position to the cranial base, and revascularization options were generalized based on the typing, primarily including aneurysmoplasty, patch angioplasty, and Vein or Dacron-graft interposition [2]. Despite the success of open surgery, intraoperative protection of cranial nerves from injury has become a stronger concern; moreover, aneurysms near the cranial base have become a new challenge because of the difficulty in exposing the operative field.
The advent of endovascular treatment has solved the issue of high aneurysms due to the difficulty of exposing the operative field for open repair, while protecting the cranial nerves as much as possible, allowing it to proceed under regional anesthesia, reducing the surgical invasiveness, which in turn shortens the length of hospitalization [16, 17]. A recent systematic review included 959 cases of ECAAs, of which, 750 were treated with open surgery (perioperative complication rates: cranial nerve injury 9%, stroke 4%, and death 2%), while 85 were treated with endovascular therapy without perioperative complications despite one case of restenosis [18]. Cornwall et al. reviewed 18 cases of endovascular therapy for ECAA with a mean follow-up of 338 days; all operations were technically successful, postprocedural surveillance imaging confirmed the elimination of ECAA, and all stents were maintained patent on follow-up imaging [19]. A systematic review involving 224 patients receiving endovascular therapy suggest that stenting for ECAA is technically feasible, with a high procedural success rate (92.8%), a relatively low complication rate (postprocedural endoleak, 8.1%; stroke, 1.8%; cranial nerve injury, 0.5%), a mean follow-up time of 15.4 ± 15.3 months, and an covered stent patency rate of 93.2% [13].
The principal objective in the management of ECAA is to prevent permanent neurologic impairment due to thromboembolism and thrombosis, as well as to exclude the risk of rupture. The management of ECAA has advanced with the development of surgical techniques. Complete removing of the aneurysm and reconstruction of blood flow to the target arteries has been pursued as the ideal procedure. However, there are currently no treatment guidelines or expert consensus for the treatment of ECAA. Based on the characteristics of ECAA, we designed the individualized procedure. For ECAA, we prioritized endovascular treatment, as shown in cases 1, 2, 4, 8, and 10 (Figs. 1a-f and 2d-f). For the type V patient with membranous stenosis, we placed a covered stent. For both dissecting aneurysm and pseudoaneurysms, stenting was performed with the dissection and at the rupture site, respectively, and case 1 with pseudoaneurysm combined with a coil embolization. Covered stents and stent-assisted embolization were the predominant endovascular treatments used in our center, and there is evidence suggesting that treatment with flow-directed braided stents and overlapping bare-metal stents are also safe and effective [19]. For patients with the difficulty of endovascular approach and high embolization risk but without difficulty in lesion exposure, we performed open surgeries, such as case 5,6 and 9 with end-to-end anastomosis (Fig. 1j). For patients with difficult lesion exposure and anatomical risk factors hindering the safe deployment and placement of stents (such as target artery tortuosity and thrombus in the aneurysm lumen), individualized hybrid procedures were devised, for instance, cases 3 and 7 (Figs. 1g-i and 2a-c). In case 3, we punctured the ICA retrogradely and antegradely under direct vision to establish the extravascular access, and then placed a covered stent into the appropriate position to completely isolate the aneurysm. In case 7, a covered stent was placed by direct puncture of the ipsilateral CCA, and the graft was sutured to the entire arterial wall outside the proximal neck of the aneurysm in order to prevent endoleak.
Except for case 1 of cerebral hemorrhage during the perioperative period, none of the patients experienced adverse events during the follow-up period. Cerebral hemorrhage was possibly linked to severe compression of the ICA in patients with ECAA, potentially associated with postoperative hyperperfusion syndrome [20]. Patients with severe carotid artery stenosis and compromised collateral circulation might benefit from staged angioplasty to mitigate the risk of hyperperfusion syndrome [21].
Conservative treatment of ECAAs has very rarely been reported. Welleweerd et al. proposed medication flowing the medical treatment guidelines for the most likely underlying disease, such as according to generalized atherosclerosis [12]. Patients were recommended to receive dual antiplatelet therapy for at least 3 months postoperatively in our center, and thereafter the medication regimen was adjusted according to other comorbidities.
There is scant data on natural follow-up. McCollum et al. have suggested that asymptomatic ECAA can have a conservative treatment through antiplatelet therapy or anticoagulation [22]. However, as surgical techniques have improved over the past 20 years and interventional devices have been updated, treatment options for asymptomatic ECAA may change. In our cohort, both asymptomatic patients underwent open surgery and were followed up for 3 years and 8 months and 10 months respectively without any new symptoms. Therefore, it is crucial to obtain data on the natural progression of ECAA. A prospective international registry study called the Carotid Aneurysm Registry Study (CAR) is currently conducting, and we look forward to data on the natural history, intervention outcomes, and follow-up of participants with ECAA [23].
ECAA is rare and only 10 cases have been treated in our center in the last 10 years. Although this study has limitations due to the small number of cases and retrospective nature, we have shared valuable experience and successful cases in developing personalized treatment strategies, thereby providing clinicians with insights, particularly in the current absence of standardized treatment guidelines. We anticipate larger-scale clinical studies and the establishment of ECAA guidelines.
Conclusion
Based on the characteristics of the aneurysm and target arteries, individualized invasive treatments for ECAA primarily include open surgery, endovascular treatment, and hybrid treatment, and they all appear to be safe and effective.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- ECAA:
-
Extracranial carotid artery aneurysm
- ICA:
-
Internal carotid artery
- CCA:
-
Common carotid artery
- DSA:
-
Digital subtraction angiogram
- CTA:
-
Computed tomography angiography
- CAR:
-
Carotid Aneurysm Registry Study
References
Gao X, Li Y, Meng L, et al. Extra-Luminal Treatment for an Internal Carotid Artery Aneurysm with Hostile Anatomic Condition. Ann Vasc Surg. 2020;66:669.e17–669.e20. https://doi.org/10.1016/j.avsg.2020.01.080.
Attigah N, Külkens S, Zausig N, et al. Surgical Therapy of Extracranial Carotid Artery Aneurysms: Long-Term Results over a 24-Year Period. Eur J Vasc Endovasc Surg. 2009;37(2):127–33. https://doi.org/10.1016/j.ejvs.2008.10.020.
Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. J Vasc Surg. 1991;13(3):452–8. https://doi.org/10.1067/mva.1991.26737.
Faggioli G, Freyrie A, Stella A, et al. Extracranial internal carotid artery aneurysms: Results of a surgical series with long-term follow-up. J Vasc Surg. 1996;23(4):587–95. https://doi.org/10.1016/S0741-5214(96)80037-1.
Donas KP, Schulte S, Pitoulias GA, Siebertz S, Horsch S. Surgical outcome of degenerative versus postreconstructive extracranial carotid artery aneurysms. J Vasc Surg. 2009;49(1):93–8. https://doi.org/10.1016/j.jvs.2008.08.006.
Fankhauser GT, Stone WM, Fowl RJ, et al. Surgical and medical management of extracranial carotid artery aneurysms. J Vasc Surg. 2015;61(2):389–93. https://doi.org/10.1016/j.jvs.2014.07.092.
Welleweerd JC, De Borst GJ. Extracranial Carotid Artery Aneurysm: Optimal Treatment Approach. Eur J Vasc Endovasc Surg. 2015;49(3):235–6. https://doi.org/10.1016/j.ejvs.2014.11.007.
Martins De Souza N, Vikatmaa P, Tulamo R, Venermo M. Etiology and treatment patterns of ruptured extracranial carotid artery aneurysm. J Vasc Surg. 2021;74(6):2097–2103.e7. https://doi.org/10.1016/j.jvs.2021.06.023
Zhou W, Lin PH, Bush RL, et al. Carotid artery aneurysm: Evolution of management over two decades. J Vasc Surg. 2006;43(3):493–6. https://doi.org/10.1016/j.jvs.2005.11.023.
Radak Đ, Davidović L, Vukobratov V, et al. Carotid Artery Aneurysms: Serbian Multicentric Study. Ann Vasc Surg. 2007;21(1):23–9. https://doi.org/10.1016/j.avsg.2006.10.004.
Mishra A, Jain N, Bhagwat A. CT Angiography of Peripheral Arterial Disease by 256-Slice Scanner: Accuracy, Advantages and Disadvantages Compared to Digital Subtraction Angiography. Vasc Endovascular Surg. 2017;51(5):247–54. https://doi.org/10.1177/1538574417698906.
Welleweerd JC, Den Ruijter HM, Nelissen BGL, et al. Management of Extracranial Carotid Artery Aneurysm. Eur J Vasc Endovasc Surg. 2015;50(2):141–7. https://doi.org/10.1016/j.ejvs.2015.05.002.
Li Z, Chang G, Yao C, et al. Endovascular Stenting of Extracranial Carotid Artery Aneurysm: A Systematic Review. Eur J Vasc Endovasc Surg. 2011;42(4):419–26. https://doi.org/10.1016/j.ejvs.2011.05.008.
El-Sabrout R, Cooley DA. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg. 2000;31(4):702–12. https://doi.org/10.1067/mva.2000.104101.
Welleweerd JC, Moll FL, De Borst GJ. Technical options for the treatment of extracranial carotid aneurysms. Expert Rev Cardiovasc Ther. 2012;10(7):925–31. https://doi.org/10.1586/erc.12.61.
Longo GM, Kibbe MR. Aneurysms of the Carotid Artery. Semin Vasc Surg. 2005;18(4):178–83. https://doi.org/10.1053/j.semvascsurg.2005.09.002.
Schechter MA, Shortell CK, Scarborough JE. Regional versus general anesthesia for carotid endarterectomy: The American College of Surgeons National Surgical Quality Improvement Program perspective. Surgery. 2012;152(3):309–14. https://doi.org/10.1016/j.surg.2012.05.008.
Hoffman ME, Squiers JJ, Hamandi M, Lanfear AT, Calligaro KD, Shutze WP. Systematic Review of the Influence of Anatomy and Aneurysm Type on Treatment Choice and Outcomes in Extracranial Carotid Artery Aneurysms. Ann Vasc Surg. 2022;83:349–57. https://doi.org/10.1016/j.avsg.2022.02.006.
Cornwall JW, Png CYM, Han DK, Tadros RO, Marin ML, Faries PL. Endovascular techniques in the treatment of extracranial carotid artery aneurysms. J Vasc Surg. 2021;73(6):2031–5. https://doi.org/10.1016/j.jvs.2020.06.133.
Abou-Chebl A, Yadav JS, Reginelli JP, Bajzer C, Bhatt D, Krieger DW. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting. J Am Coll Cardiol. 2004;43(9):1596–601. https://doi.org/10.1016/j.jacc.2003.12.039.
Hayakawa M, Sugiu K, Yoshimura S, et al. Effectiveness of staged angioplasty for avoidance of cerebral hyperperfusion syndrome after carotid revascularization. J Neurosurg. 2020;132(1):51–61. https://doi.org/10.3171/2018.8.JNS18887.
McCollum CH, Wheeler WG, Noon GP, DeBakey ME. Aneurysms of the extracranial carotid artery. Am J Surg. 1979;137(2):196–200. https://doi.org/10.1016/0002-9610(79)90144-2.
Welleweerd JC, Bots ML, Kappelle LJ, et al. Rationale and design of the extracranial Carotid artery Aneurysm Registry (CAR). J Cardiovasc Surg (torino). 2018;59(5):692–8. https://doi.org/10.23736/S0021-9509.16.08637-7.
Acknowledgements
Not applicable.
Funding
National Key Research and Development Program of China [2021YFC2500500].
Author information
Authors and Affiliations
Contributions
Conception and design: XY.G. and JL.G.; Provision of study materials or patients: Z.T. and XX. G.; Data interpretation: LR.G. and YQ.G.; Manuscript writing: All authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by The Ethics Committee of Xuan Wu Hospital of the Capital Medical University. Informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, X., Guo, J., Tong, Z. et al. Invasive treatment for extracranial carotid artery aneurysm: a single-center case series and literature review. BMC Surg 24, 221 (2024). https://doi.org/10.1186/s12893-024-02517-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-024-02517-w