Abstract
Purpose
The aim of this study was to present our initial experience and prove the feasibility of total intracorporeal laparoscopic ileal ureter replacement (TILIUR) in a single position for ureteral stricture based on membrane anatomy.
Materials and methods
Between January 2021 and April 2023, six patients underwent TILIUR in a single position for ureteral strictures based on membrane anatomy. All patients with a past medical history underwent radical hysterectomy with bilateral pelvic lymph node dissection as well as extensive ureteral stricture due to radiotherapy. The procedure is performed completely laparoscopically. Dissection of the digestive system as well as ureteral stricture or renal pelvis is based on membrane anatomy. The surgery is performed in a single position.
Results
TILIUR in a single position for ureteral stricture based on membrane anatomy was successfully performed without open conversion in all patients. Among the 6 patients, 3 patients underwent combined ileal ureter replacement (IUR) and abdominal wall ostomy, 2 underwent unilateral IUR, and 1 underwent bilateral IUR. The mean length of the ileal substitution was 22.83 cm (range: 15–28). The average operative time was 458 ± 72.77 min (range 385–575 min), and the average intraoperative blood loss was 158 mL (range 50–400 mL). The median postoperative hospital stay was 15.1 d (range: 8–32). The median duration of postoperative follow-up was 15 months (range: 3–29 months). The success rate was 100%.
Conclusions
TILIUR in a single position may be a promising option for ureteral stricture based on membrane anatomy in selected patients. Moreover, it has a positive effect on patients with renal insufficiency and urinary incontinence. Although IUR is difficult and risky, proficient surgeons can perform the procedure safely and effectively.
Similar content being viewed by others
Introduction
Cervical cancer (CC) is the fourth most common cancer among women worldwide, and radical treatment techniques include radical surgery and/or radiation. There is a high prevalence of adjuvant radiation therapy in CC [1, 2]; however, adjuvant radiation therapy significantly increases the risk of serious complications due to damage to the targeted tissues (pathological and normal) [3]. The treatment of radiation therapy-induced multisegmental strictures and even bilateral extensive ureteral strictures is more difficult and challenging due to the severe fibrosis and distortion of the anatomical planes caused by radiotherapy [4,5,6].
We previously reported that the membrane dissection concept of surgery has the advantage of a clear operative field with the protection of the ureteral blood supply by sheathing the free ureter [7]; here, we further applied the membrane dissection concept to the more complex situation of radiation-induced extensive ureteral stricture (RIEUS). The ileum is suitable as an alternative to the ureter because of its relatively rich blood supply and peristaltic nature [8]. Ileal ureter replacement (IUR) was first reported by Shoemaker in 1906 and was later popularized by Goodwin et al. [9] in the late 1950s.
Currently, most ileal substitution procedures are performed with the assistance of robotic systems, and a few are performed completely intracorporeally. In addition, data on total intracorporeal laparoscopic ileal ureter replacement (TILIUR) are poor and mainly limited to case reports. The present study reports our TILIUR technique in a single position, performed in six consecutive patients, and compares it with prior experiences available in the literature.
Methods
Patients
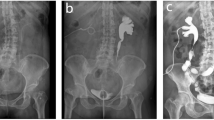
From January 2021 to April 2023, 6 patients treated with TILIUR in a single position were enrolled. The clinical data, including demographics, gynaecological cancer histories, perioperative records, imaging studies, complications, and follow-ups, were retrospectively collected. Subjective standards were used to make the diagnosis, such as flank pain, repeated fever and radiological criteria, including abdominal ultrasonography, retrograde urography or antegrade + retrograde urography, computed tomography urography (CTU) and magnetic resonance urography(MRU). All patients had ureteral stents placed and replaced regularly for strictures.According to our definition, the main surgical indication for TILIUR was patients with bilateral long or multiple ureteral strictures. TILIUR combined with abdominal wall ostomy is indicated in patients with bladder dysfunction, urinary incontinence, or preoperative creatinine greater than 2 mg/dL.Preoperative images of the patient with ureteral strictures are shown in Fig. 1.The Clavien‒Dindo classification system was used to evaluate the complications. Jinan University’s Affiliated Hospitals’ Ethics Committee (first and sixth hospitals) authorised this research. Informed consent was obtained from all participants.
Imaging of the patient’s strictures prior to the surgery (A) MRU showed the mid-distal stricture of bilateral ureter, (B) Three-dimensional reconstruction of CTU showed long obliterating stricture of left tract and twisted upper right ureter with stricture, (C) Three-dimensional reconstruction of CTU showed long obliterating stricture of bilateral tract, (D) MRU showing left mid-ureteral stricture and right upper and mid-ureteral stricture, (E) Three-dimensional reconstruction of CTU showed mid-right ureteral stricture with indwelling ureteral stenting, (F) Three-dimensional reconstruction of the CTU showed the mid-distal stricture of right ureter
Surgical technique
Preoperative preparation
Patients had a nephrostomy on the afflicted side one month before surgery to drain urine and protect renal function.Preoperatively, patients were routinely treated with third-generation cephalosporins for anti-infection treatment, which was adjusted after urine culture results were obtained if necessary. Patients were placed on a liquid diet for 2 days prior to surgery, the intestines were cleaned the day before the surgery with polyethylene glycol electrolyte, and enteral nutrition emulsions were taken to guarantee a scum-free diet.
Position and trocar distribution
After induction of general anaesthesia, a 20# three-way catheter was inserted. Each patient was placed in a 30° Trendelenburg tilt with both lower limbs spread outwards at approximately 60°. A five-port approach was used, and port placement is shown in Fig. 2A and B. A port (10-mm trocar) was placed midway between the umbilicus and the symphysis pubis (Fig. 2A) and midway between the umbilicus and the subxiphoid process (Fig. 2), and then a 30° camera was placed. The caudal approach was used for TILIUR combined with abdominal wall ostomy in a single position (Fig. 2A), and the cephalad approach was used in a single position for TILIUR (Fig. 2B).
Surgical procedures
Dissection of the digestive system, ureteral stricture or renal pelvis based on membrane anatomy
Caudal approach
To expose the posterior peritoneum, the distal ileum was moved to the cephalic side. Then, the posterior peritoneum was incised along with the ileocecal and small bowel mesenteric roots at the fusion with the posterior abdominal wall. Dissection was performed by entering the level between the posterior mesenteric lobe and the anterior renal fascia, reaching the level of the right renal hilum. Subsequently, the renal fascia covering the surface of the right ureter was opened, the upper ureter was freed along the right ureteral sheath, and the distal ureter was clamped with Hem-o-lock clips at the ureteral stenosis. Then, the peritoneum was incised medially in the inferior mesenteric vein, and the level of the left renal hilum was revealed along with the level between the posterior lobe of the descending mesentery and the anterior lobe of the left renal fascia. The upper part of the left ureter was freed in the same way, and the distal stenotic ureter was clamped. Sterile saline (0.9%) was injected into the nephrostomy tube on the affected side to fill the urinary system to assess the proximal dilated ureter and dilated renal pelvis. By observing whether sterile saline flowed smoothly out of the proximal end of the ureter, the level of a healthy ureter was judged. Finally, the length of the ureteral defect was measured.
Cephalad approach
Dissection of the digestive system and ureteral stricture via the cephalad approach was similar to that in our previously reported study [7]. On the left (or right) side, dissection was performed along the level between the posterior lobe of the left (or right) colonic mesentery and the anterior layer of the anterior renal fusion fascia, and the descending colon, the splenic flexure of the colon, the tail of the pancreas, or the right ascending colon was moved to the opposite side. Ureterolysis was carried out until proximal dilated normal ureteral tissue was encountered. If adjacent tissue adhesion was encountered, blunt and sharp dissection of dense adhesions was meticulously carried out, and the distal end of the dilated ureter was clamped with Hem−o−lock clips. The length of the ureteral defect was measured in the same way.
Preparation of ileal ureter substitutes
After measuring the length of the ureteral defect, a 20–30 cm ileal segment was marked and dissected using 3 − 0 absorbable thread at least 15 cm from the ileocecal region for backup (Fig. 3A). A longitudinal side-to-side anastomosis of the mesenteric rim was then performed using an Endo-GIA stapler (ECR60W, Ethicon Endo-Surgery, USA) to restore ileal continuity (Fig. 3B). Next, the proximal and distal ileum were anastomosed transversely with the assistance of an Endo-GIA stapler (Fig. 3C). Finally, the anastomotic edges were sutured interruptedly (Fig. 3D).
Reconstruction of the ileal ureter replacement
The ileal ureteral replacement in this study was categorized into three different types.
An F6 DJ tube was put in the left ureter after an end-to-end anastomosis between the proximal ileum and the left ureter using a 3 − 0 absorbable barbed suture.Another F6 DJ tube was placed into the ureter following side-to-side anastomosis between the right ureter and the middle ileum. Approximately 3 cm to the right of the umbilicus, a 2 cm diameter abdominal wall ostomy was made. The distal ileum was pulled out from the body and then twisted and sutured into a papilla shape before being implanted in the abdominal wall. Finally, an F22 latex catheter was left in the ileum(Fig. 4A).
In the bilateral IUR, called the reverse 7-shaped IUR, the proximal ileum was anastomosed end to end with the left ureter, the right ureter was anastomosed side to side with the ileum, and the distal ileum was twisted and sutured into a papilla shape and then implanted into the bladder (Fig. 4B).
For unilateral IUR, the proximal end of the ileum was end-to-end anastomosed with the right ureter, and the distal end of the ileum was twisted and sutured into a papilla shape and finally implanted into the bladder (Fig. 4C).
The tension-free, watertight, and full-thickness surgical principles were applied to all end-to-end anastomoses without torsion of the mesentery. A drainage tube was placed near the uretero-ileal anastomosis after confirming that the ureter was unobstructed and tightly anastomosed. The surgical incision was then stitched up.
Follow‑up
Patients were followed up at 1, 3 and 6 months after surgery, and follow-up consisted of symptom evaluation, physical examinations, blood tests (including serum creatinine and electrolyte tests), routine urine tests, urological ultrasound, and CTU. The drain was removed when drainage was reduced to < 10 mL/day. Two weeks after the surgery, the three-way catheters were removed. DJ tubes were removed through cystoscopy two months after surgery. Surgical success was defined as the alleviation of subjective symptoms and the improvement of hydronephrosis.
Results
A total of 6 female patients underwent TILIUR by the same surgeon between January 2021 and April 2023. All patients with medical history had radical hysterectomy and bilateral pelvic lymph node dissection. Table 1 shows patient demographics. The mean age was 56.7 y (range: 41–69). The main indication for ureteral replacement in our series was extensive ureteral strictures caused by radiotherapy after surgery for cervical cancer, with 4 cases of bilateral extensive ureteral strictures and 2 cases of right extensive ureteral strictures. Among the six patients, one patient each had flank pain as well as urinary incontinence with fever or haematuria, two had fever, and one patient each had flank pain and no symptoms. The mean time interval from radiotherapy to ureteral stricture detection was 23.7 months (range: 2–60).
All patients had a double-J stent and percutaneous nephrostomy for preoperative ureteral stricture.Table 2 shows patient surgery and follow-up data. The mean length of the ileal substitution was 22.83 cm (range: 15–28). The average operative time was 458 ± 72.77 min (range 385–575 min), and the average intraoperative blood loss was 158 mL (range 50–400 mL). Among the 6 patients, 3 patients received combined IUR and abdominal wall ostomy, 2 received unilateral IUR, and 1 received bilateral IUR. Each type of IUR is shown in Fig. 3. The median postoperative hospital stay was 15.1 d (range: 8–32). The median duration of postoperative follow-up was 15 months (range: 3–29 months). Regarding postoperative complications, one patient experienced incomplete ileus, which was resolved after conservative treatment, and one patient had retraction of the abdominal wall stoma due to abdominal obesity. In total, 6 patients had a decreased or stable hydronephrosis.The average creatinine levels before surgery and at the last follow-up visit were 1.78 (0.75–3.69) mg/dL and 1.62 (0.86–2.90) mg/dL, respectively. Renal function improved or remained stable in all 6 patients (Fig. 5).
Discussion
The ischaemia and fibrosis of the ureter and surrounding tissues caused by radiotherapy is a great challenge for surgeons. Long ureteral defects are treated surgically by constructing a nonrefluxing and nonobstructed urine outflow as quickly as feasible to restore or stabilize renal function [10].
In our case series, all patients had ureteral stents placed and replaced regularly for strictures. However, long-term indwelling ureteral stents may cause recurring urinary tract infections and renal function issues, putting patients under severe emotional, psychological, and financial stress.It is well known that the treatment of extensive long-segment ureteral strictures includes uretero-ureterostomy, renal autotransplantation, Boari bladder flap, and IUR. Historically, IUR was performed for ureteral strictures secondary to tuberculosis. However, among all available treatment options for radiation-induced extensive ureteral strictures, IUR is the last and only viable option at this time. Park JJ et al. [11]and Monn MF et al. [5] reported their experience with IUR in the treatment of radiation-induced ureteral stricture, but their procedures were performed open. Limited literature has reported completely intracorporeal laparoscopic IUR, and only a few case reports have concentrated on the treatment of long ureteral strictures after radiation therapy.
For example, Kochkin A et al. [12] reported the experience of 40 cases of total intracorporeal laparoscopic IUR for the treatment of long ureteral defects, which required a change in table position during the procedure. In addition, it has been reported that the current robot used to perform IUR must be undocked and redocked during the ileovesical anastomosis stage of the procedure [4]. Furthermore, ureteral strictures caused by radiation therapy after surgery for cervical cancer are often associated with severe scarring and fibrosis of the periureteral tissue, which poses a serious challenge for the surgeon when dissecting the ureter while preserving its blood supply. To optimize the problems faced above, we innovatively proposed a single-position TILIUR, and put the concept based on membrane anatomy that we reported earlier, that is, pay more attention to the plane between tissue and tissue when dissecting the ureter, and applied this technique to the dissection of ureteral strictures after radiotherapy.
The standard treatment for early-stage cervical cancer is radical hysterectomy with bilateral pelvic lymph node dissection. The important technical step in radical hysterectomy is to perform wide dissection of the periureteral tissue and bladder [13].Thus, we can consider that the ureters, bilateral iliac vessels, and peripheral tissues of the bladder in these patients after radical hysterectomy are mostly “naked” because of bilateral pelvic lymph node dissection, dissections of the ureters, and adjuvant radiation therapy and are replaced by a covering of fibrous scar tissue. It is difficult to free the fibrous scar tissue covering the surface of the ureter, and an alternative strategy was employed in our study series by looking for normal tissue with space as an entry point and by a combination of blunt and sharp methods if severe fibrous scar tissue was encountered.
Thereare limited TILIUR reports for treating postoperative cervical cancer radiotherapy-induced ureteral strictures. Gözen AS et al. [12] reported 40 cases of TILIUR in 2020, one of which was for ureteral strictures caused by cervical surgery followed by radiotherapy, and the mean operative time in this series was 335 (150–680) minutes, with a mean estimated blood loss of 221 (50–400) ml; Li B et al. [14]reported two cases in 2021, with operative times of 420 min and 410 min per patient and an estimated blood loss of 120 ml and 100 ml, respectively; Li X et al. [15] reported 15 cases of robotic-assisted total intracorporeal robot-assisted ileal ureter replacement (RA-IUR) in 2023, with 7 patients treated with unilateral RA-IUR and 8 patients treated with bilateral RA-IUR, with a mean operative time of 261.8 min (183–381 min) and an estimated blood loss of 64.7 ml (30–100 ml), including 7 patients who had surgery for cervical cancer plus radiotherapy. Our series were all in this category, with a mean operative time of 458 min (385–575 min) and an estimated blood loss of 158 ml (50–400 ml). Although the operation time was slightly longer and the estimated blood loss was slightly higher, in terms of postoperative outcome, the results were generally consistent with the above studies. One patient with postoperative incomplete ileus recovered following conservative therapy, one with abdominal wall stoma retraction due to obesity, and the others experienced no complications.
Next, in terms of indications for surgery, the main surgical indication for TILIUR is patients with bilateral long or multiple ureteral strictures, which is similar to the surgical indications reported in the current literature [15]. IUR combined with abdominal wall stoma is recommended for patients with bilateral long or multiple ureteral strictures accompanied by bladder dysfunction, such as urinary incontinence, or preoperative creatinine greater than 2 mg/dl. Because gynaecological cancer surgery and radiotherapy can cause complications of low-compliance bladder and vesicovaginal fistula, in this case, it can cause repeated urinary tract irritation or urinary incontinence. To maintain kidney function and improve lower urinary tract symptoms at the same time, urinary tract diversion is inevitable [16, 17].
Although the mid-urethral sling is the gold standard for treating urinary incontinence in women [18], for the loss of bladder capacity and urethral sphincter function that cannot be repaired, abdominal wall ostomy is an appropriate and feasible surgical method.
A previous study also supports the fact that half of the patients with serum creatinine over 2 mg/dl develop hyperchloremic metabolic acidosis, and the procedure needs to be changed to the conduit [19]. However, a study by Armatys SA et al. [20]showed that six patients had preoperative baseline creatinine greater than 2.0 mg/dl, and renal function stabilized or improved in five cases; therefore, we believe that IUR in patients with preoperative creatinine greater than 2.0 mg/dl still has a role in stabilizing renal function. Impaired renal function was not a contraindication to surgery, according to studies, and IUR may help preserve renal function in patients with high serum creatinine (> 2 mg/dl) [21, 22]. In our case, two patients with preoperative creatinine greater than 2.0 mg/dl had a decrease in creatinine at the last follow-up compared with the preoperative period.
Based on our encouraging initial experience, TILIUR in a single position based on membrane anatomy may be a promising option for the treatment of ureteral strictures induced by radiotherapy for cervical cancer. There are certain considerations in the management of RIEUS based on our experience. First, for patients with acute renal failure caused by RIEUS, preoperative percutaneous nephrostomy protects renal function and reduces the risk of urinary tract infection as well as effectively decreasing the incidence of postoperative urinary leakage and improving the healing of the ureter-ileum anastomosis; moreover, nephrostomy can be used intraoperatively to observe saline flow to assess the health of the ureter. Second, for patients with RIEUS accompanied by renal insufficiency and bladder dysfunction, TILIUR combined with abdominal wall ostomy may be considered because of the positive effect on the protection of renal function. All anastomoses adhered to the surgical principles of being tension free and waterproof and protecting the blood supply. Therefore, this requires proficiency in laparoscopic techniques as well as extensive experience in ureteral reconstruction for the urologist. Third, for dissection of the bilateral renal pelvis and upper ureter, we used a single caudal approach. This approach offers the advantages of avoiding procedures such as changing positions, resterilization, and draping during the surgery as well as simplifying the process and significantly reducing the operating time.
Although encouraging, the results of our report must be considered in the context of its limitations. First, all of the procedures were carried out by surgeons who were well trained and had much expertise with laparoscopic surgery. As a result, our findings could not be applied to all surgeons. Additionally, this was a pilot study, and the sample size was limited, which might impact the results. Therefore, to confirm our preliminary findings, more research with larger populations and longer follow-ups is needed.
Conclusions
TILIUR in a single position for ureteral stricture based on membrane anatomy may be a promising option for selected patients. Moreover, it has a positive effect on patients with renal insufficiency and urinary incontinence. Although IUR is difficult and risky, proficient surgeons can perform the procedures safely and effectively.
Data availability
All data generated or analysed during this study are included in this article.
Abbreviations
- TILIUR:
-
Total intracorporeal laparoscopic ileal ureter replacement
- IUR:
-
Ileal ureter replacement
- CC:
-
Cervical cancer
- RIEUS:
-
Radiation-induced extensive ureteral stricture
- CTU:
-
Computed tomography urography
References
Ghezzi F, Cromi A, Serati M, et al. Radiation-induced bowel complications: laparoscopic versus open staging of gynecologic malignancy. Ann Surg Oncol. 2011;18(3):782–91.
Landoni F, Sartori E, Maggino T, et al. Is there a role for postoperative treatment in patients with stage Ib2-IIb cervical cancer treated with neo-adjuvant chemotherapy and radical surgery? An Italian multicenter retrospective study. Gynecol Oncol. 2014;132(3):611–7.
Bogani G, Cromi A, Serati M, et al. Predictors of postoperative morbidity after laparoscopic versus open radical hysterectomy plus external beam radiotherapy: a propensity-matched comparison. J Surg Oncol. 2014;110(7):893–8.
Grosso AA, Di Maida F, Mari A, et al. Totally intracorporeal robotic ileal ureter replacement: focus on surgical technique and outcomes. Minerva Urol Nefrol. 2021. https://doi.org/10.23736/S0393-2249.20.04191-0.
Monn MF, Roth JD, Bihrle R, Mellon MJ. Long term outcomes in the use of ileal ureter for radiation-induced ureteral strictures. Int Urol Nephrol. 2018;50(8):1375–80.
Asghar AM, Lee Z, Lee RA, et al. Robotic Ureteral Reconstruction in patients with Radiation-Induced Ureteral strictures: experience from the collaborative of reconstructive robotic ureteral surgery. J Endourol. 2021;35(2):144–50.
Wu G, Li H, Zhong P, et al. Initial experience with the Comprehensive modified laparoscopic pyeloplasty technique based on membrane anatomy for treating Ureteropelvic Junction obstruction. Urol Int. 2022;106(5):487–94.
Sim A, Todenhöfer T, Mischinger J, et al. Intracorporeal ileal ureter replacement using laparoscopy and robotics. Cent Eur J Urol. 2014;67(4):420–3.
GOODWIN WE, WINTER CC. Replacement of the ureter by small intestine: clinical application and results of the ileal ureter. J Urol. 1959;81(3):406–18.
Zhong W, Hong P, Ding G, et al. Technical considerations and outcomes for ileal ureter replacement: a retrospective study in China. BMC Surg. 2019;19(1):9. Published 2019 Jan 18.
Park JJ, Yun S, Kim JH. Bilateral ureteral replacement using ileum in bilateral refractory ureteral stricture with renal insufficiency: a case report and review of literature. Transl Androl Urol. 2020;9(3):1466–74.
Kochkin A, Tokas T, Gallyamov E, et al. Laparoscopic totally intracorporeal ileal ureter replacement: a multi-institutional study. Minim Invasive Ther Allied Technol. 2022;31(1):119–26.
Hwang JH, Kim BW. Laparoscopic radical hysterectomy has higher risk of perioperative urologic complication than abdominal radical hysterectomy: a meta-analysis of 38 studies. Surg Endosc. 2020;34(4):1509–21.
Liang C, Chai S, Gao X, et al. Laparoscopic reverse 7 ileal ureteral replacement for bilateral extensive ureteral strictures performed completely intracorporeally: the initial experience. Int Urol Nephrol. 2021;53(5):919–24.
Yang K, Wang X, Xu C et al. Totally Intracorporeal Robot-assisted unilateral or bilateral Ileal ureter replacement for the treatment of Ureteral strictures: technique and outcomes from a single center [published online ahead of print, 2023 May 22]. Eur Urol. 2023;S0302-2838(23)02784-7.
Wit EM, Horenblas S. Urological complications after treatment of cervical cancer. Nat Rev Urol. 2014;11(2):110–7.
Knight RB, Hudak SJ, Morey AF. Strategies for open reconstruction of upper ureteral strictures. Urol Clin North Am. 2013;40(3):351–61.
Krhut J, Martan A, Jurakova M, Nemec D, Masata J, Zvara P. Treatment of stress urinary incontinence using polyacrylamide hydrogel in women after radiotherapy: 1-year follow-up. Int Urogynecol J. 2016;27(2):301–5.
Koch MO, McDougal WS. Nicotinic acid: treatment for the hyperchloremic acidosis following urinary diversion through intestinal segments. J Urol. 1985;134(1):162–4.
Armatys SA, Mellon MJ, Beck SD, Koch MO, Foster RS, Bihrle R. Use of ileum as ureteral replacement in urological reconstruction. J Urol. 2009;181(1):177–81.
Poujade L, Branchereau J, Rigaud J, Perrouin-Verbe MA. Ileal ureter replacement: early morbidity and long-term results [published online ahead of print, 2020 Nov 11]. Prog Urol. 2020;S1166-7087(20)30605-9.
Pamecha Y, Shelke U, Patil B, Patwardhan S, Kini S. Use of ileum for complex ureteric reconstruction: Assessment of long-term outcome, complications, and impact on renal function. Urol Ann. 2018;10(4):369–74.
Acknowledgements
The authors are grateful to Dr GW, who is also an outstanding medical illustrator, and the entire staff of the Department of Urology at the Sixth Affiliated Hospital of Jinan University, as well as the First Affiliated Hospital of Jinan University.We would like to thank AJE (www.aje.com) for the linguistic assistance during the preparation of this manuscript.
Funding
This study was supported by the Medical Scientific Research Foundation of Guangdong Province, China (A2022186 to Z.H.C.), the Science and Technology Project of the Health Bureau of Yangjiang City (2021036 to C.Y.L., SF2021212 to C.Y.L.), the Key Projects of Science and Technology of Social Development in Dongguan City (20231800935912 to C.Y.L.), the Introduction and Training Project of High-level Health Talents in Dongguan City, and the High-level Talent Start-up Project of the Sixth Affiliated Hospital of Jinan University.
Author information
Authors and Affiliations
Contributions
G.W.: manuscript writing, acquisition of data, analysis and interpretation of the data. H.L.: manuscript writing, supervision. F.L., H.Z., and Y.Y.G.: assistance with data collection. L.X., H.L., and Z.C.: manuscript review. D.Y. and C.L.: project development, management, operations, supervision. All authors agree to all aspects of the work.
Corresponding authors
Ethics declarations
Ethical statement
This study was approved by the Ethics Committee of the First and Sixth Hospital of Jinan University. Written informed consent was obtained from each patient.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, G., Li, H., Luo, F. et al. Total intracorporeal laparoscopic ileal ureter replacement in a single position for ureteral stricture based on membrane anatomy. BMC Surg 24, 88 (2024). https://doi.org/10.1186/s12893-024-02363-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-024-02363-w