Abstract
Background
To evaluate the impact of tumor size on the perioperative and long-term outcomes of liver resection for hepatocellular carcinoma (HCC).
Methods
We reviewed the patients’ data who underwent liver resection for HCC between November 2009 and 2019. Patients were divided into 3 groups according to the tumor size. Group I: HCC < 5 cm, Group II: HCC between 5 to 10 cm, and Group III: HCC ≥ 10 cm in size.
Results
Three hundred fifteen patients were included in the current study. Lower platelets count was noted Groups I and II. Higher serum alpha-feto protein was noted in Group III. Higher incidence of multiple tumors, macroscopic portal vein invasion, nearby organ invasion and presence of porta-hepatis lymph nodes were found in Group III. More major liver resections were performed in Group III. Longer operation time, more blood loss and more transfusion requirements were found in Group III. Longer hospital stay and more postoperative morbidities were noted in Group III, especially posthepatectomy liver failure, and respiratory complications.
The median follow-up duration was 17 months (7–110 months). Mortality occurred in 100 patients (31.7%) and recurrence occurred in 147 patients (46.7%). There were no significant differences between the groups regarding recurrence free survival (Log Rank, p = 0.089) but not for overall survival (Log Rank, p = 0.001).
Conclusion
HCC size is not a contraindication for liver resection. With proper selection, safe techniques and standardized care, adequate outcomes could be achieved.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is one of the commonest malignancies worldwide, and the third most common cause of cancer-related death. It is the most common primary malignancy affecting the liver [1,2,3]. Liver resection and transplantation are the only available curative treatment lines for HCC patients. Owing to several limitations of liver transplantation, liver resection is considered the treatment of choice for HCC patients with solitary tumor and sufficient hepatic functional reserve. With the refinement of the surgical techniques, and advancement of perioperative care, the outcomes of liver resection have markedly improved in the recent years [4, 5]. On the other hand, local thermal ablation especially if combined with immunotherapy could provide effective control of the tumor with acceptable survival outcomes [6, 7].
Several factors had been identified to predict poor outcomes after curative liver resection for HCC as tumor size, number, presence of vascular invasion, tumor differentiation and serum tumor markers [8]. The modified American Joint Committee on Cancer (AJCC) tumor-lymph node-metastasis system (pTNM) includes tumor size, number, and vascular invasion in its tumor (T) classification. Therefore, tumor size should be considered as one of the most important predictive factors for tumor recurrence and patient survival [4, 9]. Also, it is known that the prognosis of patients undergoing curative resection for larger HCC is inferior to those with small HCC. This is attributed to the fact that larger HCCs are frequently associated with some adverse prognostic factors like the presence of microscopic vascular invasion [10, 11]. On the other hand, some studies from high-volume centers had shown the tumor size did not affect survival outcomes in patients who underwent liver resection for single HCC without vascular invasion [12, 13].
The current study was conducted to evaluate the impact of tumor size on the outcomes of liver resection for HCC among Egyptian patients, in area where hepatitis C virus (genotype 4) is the main predisposing factor for HCC development and evaluate the prognostic impact of tumor size on the long-term survival outcomes.
Methods
Study design
We retrospectively review the data of patients who underwent primary liver resection for HCC at Gastro-intestinal Surgery Center (GISC), Mansoura University, Egypt during the period between November 2009 and November 2019. Patients were divided into 3 groups according to the tumor size in the final pathological report. Group I included patients with tumors < 5 cm in size, Group II included patients with tumors between 5 to 10 cm in size, and Group III included patients with tumors ≥ 10 cm in size.
Patient data were retrieved from a prospectively maintained database for all patients undergoing liver resection. An informed consent was obtained from each patient prior to surgical intervention. The study was approved by the Institutional Review Board and Local Ethical Committee at the Faculty of Medicine, Mansoura University, Egypt (R.20.06.874). The current study methods were carried out in accordance with the Declaration of Helsinki.
Preoperative evaluation
Preoperative workup included detailed clinical, laboratory, and radiological evaluation was described before [8, 14]. Generally, liver resection was applied for patients with preserved liver functions (i.e., sufficient future liver remnant), without signs of severe portal hypertension, without evidence of extrahepatic metastasis, and with American Society of Anesthesiologists (ASA) grade < III [15].
Surgical procedure
The surgical procedure had been described elsewhere [8, 14]. The types of liver resection were defined according to Brisbane 2000 terminology [16]. Generally, parenchymal sparing liver resection was preferred for fear of postoperative liver dysfunction. Major liver resections were performed for patients with large tumors or tumors close to major hepatic vasculature if the future remnant liver is adequate (more than 40% of the total liver volume). Volumetric assessment was performed for selected patients requiring major liver resection with marginal liver functions. Otherwise, non-anatomical liver resections were more preferred. Liver parenchymatous transection was performed by combinations of clamp-crush method and ultrasonic devices. Intermittent Pringle’s maneuver was applied selectively during liver transection. Intraoperative ultrasonography (Canon, Xario 200, Japan) was utilized in some patients to check the resection margin and exclude presence of multifocal tumors [8, 14].
Postoperative care and follow up
After surgery, patients were transferred to the intensive care unit or to the ward for monitoring. All patients underwent daily laboratory evaluation. Abdominal ultrasonography was performed routinely in all patients. Oral fluids were started once intestinal sounds are restored. Abdominal drains were removed when daily output was less than 100 cc with absence of any abdominal collections [8, 14].
After discharge, patients were followed-up in the outpatient clinic. Follow-up visit included physical examination, serum liver function tests, serum alpha fetoprotein, abdominal ultrasonography, and triphasic computed tomography when recurrence was suspected [8, 14].
Definitions
Postoperative morbidity is defined as adverse events happening during the early postoperative period and is graded according to the Clavien-Dindo classification [17]. Postoperative liver dysfunction, biliary fistula and hemorrhage are defined according to the ISGLS definitions [18,19,20]. Early postoperative mortality was defined as mortality occurring during the first 90 postoperative days and was excluded from further survival analysis.
Overall survival (OS) was calculated from the day of surgery to the day of confirmed death or the last follow up visit. Disease-free survival (DFS) was calculated from the day of surgery to the day of confirmed tumor recurrence or the day of death or last follow up.
Statistical analysis
Categorical variables were expressed as number (percentage), and continuous variables were expressed as median (range). Comparison between the three groups was done by chi-square or ANOVA test when appropriate and comparison between each two groups was done by pair-wise comparisons. Survival rates were calculated by Kaplan–Meier method, and comparison between groups was done by Log-Rank test.
Statistical analysis was performed using the SPSS 22 software (IBM, Chicago, IL, USA). A p value less than 0.05 was considered statistically significant.
Results
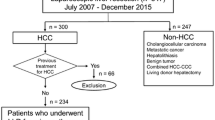
During the period between November 2009 and November 2019, 315 patients with pathologically confirmed hepatocellular carcinoma underwent liver resection at Gastrointestinal Surgery Center, Mansoura University, Egypt.
Patients were divided into 3 groups according to the tumor size. Group I included patients with tumors < 5 cm in size (88 patients – 26.7%). Group II included patients with tumors between 5 to 10 cm in size (167 patients – 50.8%). Group III included patients with tumors ≥ 10 cm in size (60 patients – 18.2%).
Demographic data
The demographic data of the study patients were summarized in Table 1. There were significant differences between the groups regarding clinical presentation, serum albumin, serum alanine aminotransferase, platelets count, serum alpha-feto protein, and hepatitis C virus antibodies. Higher incidence of accidentally discovered HCC was noted in Group I and II while more abdominal pain and masses were noted in Group III. Higher serum albumin and alanine aminotransferase were noted in Group I, while lower platelets counts was noted Groups I and II. Higher serum alpha-feto protein was noted in Group III.
Radiological and Endoscopic data
Radiological and endoscopic data of the study patients were summarized in Table 2. There were significant differences between the groups regarding liver status, tumor site and macroscopic portal vein invasion. Higher incidence of liver cirrhosis was found in Group I and II. Higher incidence of macroscopic portal vein invasion was found in Group III.
Operative data
Operative data of the study patients were summarized in Table 3. Open conversion occurred in 2 cases (1.2%) because of bleeding. There were significant differences between the groups regarding liver status, tumor site, tumor number, macroscopic vascular invasion, nearby organ invasion, presence of lymph nodes, liver resection extent, liver resection type, Pringle maneuver indication, total operation time, operative blood loss, and blood transfusion requirements.
Higher incidence of liver cirrhosis was found in Group I and II. Tumors were more commonly located in left hemi-liver in Group I and right hemi-liver in Groups II and III. Higher incidence of multiple tumors, macroscopic portal vein invasion, nearby organ invasion and presence of porta-hepatis lymph nodes were found in Group III. More major liver resections were performed in Group III. Pringle maneuver was more electively utilized in Groups I and II and emergently utilized in Group III. Longer operation time, more operative blood loss and more transfusion requirements were found in Group III.
Postoperative data
Postoperative data of the study patients were summarized in Table 4. There were significant differences between the groups regarding ICU stay, total hospital stay, morbidities, posthepatectomy liver failure (PHLF), and respiratory complications. Longer hospital stay and more postoperative morbidities were noted in Group III. Also, more common posthepatectomy liver failure, and respiratory complications were noted in Group III.
Pathological data
Pathological data of the study patients were summarized in Table 5. There were significant differences between the groups regarding tumor size, number, resection margin, microvascular invasion, perineural invasion, tumor grade, and liver background. More multiple tumors, microvascular invasion, perineural invasion were found in Group III. More R0 resections were performed in Groups I and II. Higher incidence of pathologically confirmed liver cirrhosis was found in Group I and II.
Survival outcomes
Overall survival
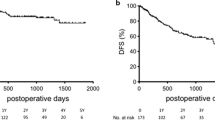
The median follow-up duration was 17 months (7–110 months). Mortality occurred in 100 patients (31.7%). The 1-, 3-, and 5-years OS rates of all study cases were 81.2%, 65.5%, and 48.3%, respectively (Fig. 1A).
The 1-, 3-, and 5-years OS rates of Group I cases were 86.2%, 67.9%, and 49.6%, respectively. The 1-, 3-, and 5-years OS rates of Group II cases were 89.4%, 67.8%, and 55.1%, respectively. The 1-, 3-, and 5-years OS rates of Group III cases were 70.7%, 37.9%, and 32.5%, respectively (Log Rank: Chi-Square = 27.4, df = 2, p = 0.001) (Fig. 2A).
Disease-free survival
Recurrence occurred in 147 patients (46.7%). There were no significant differences between the groups regarding recurrence time, and site as shown in Table 6. The 1-, 3-, and 5-years DFS rates of all study cases were 72.1%, 41.4%, and 26.5%, respectively (Fig. 1B).
The 1-, 3-, and 5-years DFS rates of Group I cases were 77%, 48.2%, and 28.6%, respectively. The 1-, 3-, and 5-years DFS rates of Group II cases were 74.2%, 38.5%, and 29.9%, respectively. The 1-, 3-, and 5-years DFS rates of Group III cases were 57.5%, 33.5%, and 11.2%, respectively (Log Rank: Chi-Square = 4.8, df = 2, p = 0.089) (Fig. 2B).
Discussion
HCC is one of the most prevalent neoplasms all over the world. HCC is one of the most lethal neoplasms worldwide and it represents the second most common cause of tumor associated mortalities [21, 22]. The condition is not much different in Egypt. The Egyptian Health Authorities considered HCC as the most challenging health care problem among Egyptians. The prevalence of HCC is continuously rising among Egyptian patients owing to the high prevalence of hepatitis C viral (HCV) infection (genotype 4) [23].
Liver resection is one of the curative lines of HCC patients especially in patients with early HCC with well-preserved liver functions with acceptable perioperative and long-term outcomes [24, 25]. On the other hand, the presence of liver cirrhosis may lead to higher incidence of perioperative morbidity and mortality with poorer long-term survival outcomes [26]. The current study was conducted to review our center experience of liver resection for HCC and evaluate the prognostic impact of tumor size on the outcomes of liver resection for HCC among Egyptian patients, in area where hepatitis C virus (genotype 4) is the main predisposing factor for HCC development.
Tumor size is an important predictive factor for the outcomes after surgical treatment for HCC. This might be attributed to the fact that larger HCCs are frequently associated with some adverse prognostic factors like the presence of microscopic vascular invasion. Also, it is known that the prognosis of patients undergoing curative resection for larger HCC is inferior to those with small HCC [10, 11]. Therefore, tumor size should be considered as one of the most important predictive factors for tumor recurrence and patient survival [4, 9]. In general practice, there is no size limitation regarding liver resection for HCC if the remnant liver volume is adequate. This is not clearly matched with the recent practice guidelines of BCLC staging system due to the great variations between areas in social, environmental, and medical conditions [27].
Liver resection especially in cirrhotic patients is always associated with more blood loss and higher needs for perioperative transfusions. In the current study, pathological liver cirrhosis was found in 298 patients (94.6%). Previous studies had addressed that blood loss and perioperative transfusions are associated with the development of immunological reactions and nonspecific immunosuppression, which subsequently affect the development of postoperative morbidities and negatively affects the prognosis of HCC patients [28, 29]. In the current study, we noticed longer operation time, more intraoperative blood loss and more transfusion requirements among patients with larger tumors compared to counters with smaller ones. This is related to the more frequent major liver resections and more macroscopic portal vein invasion (PVI) among this group of patients.
HCC has a high affinity for macroscopic portal vein invasion (PVI). The presence of macroscopic PVI is considered a strong negative prognostic factor for HCC patients. This is attributed to the high risk of dissemination of the cancer cells into the blood stream and metastasis to to other parts of the liver and distant other organs [30, 31]. We previously reported that surgical management of selected HCC patients associated with the presence of macroscopic PVI is technically feasible and is associated with comparable recurrence free survival but poorer overall survival, when compared to a matched group of HCC patients without macroscopic PVI [14]. In the current study we found significantly higher incidence of macroscopic PVI among patients with large sized HCCs compared to smaller HCCs, especially in patients with tumors larger than 10 cm. This is denoting a strong correlation between tumor size and the presence of macroscopic vascular invasion.
In the current study, we found a higher incidence of post-operative morbidities in HCC patients with larger tumors, especially the incidence of PHLF and respiratory complications. Previous studies had shown a varying incidence of postoperative morbidities after liver resection for HCC reaching up to 70%. Differences in the incidence of post-operative morbidities are mainly related to the differences in the patient selection and background liver disease. The commonest complications encountered were postoperative bleeding, liver dysfunction or liver failure, ascites, and sepsis [32,33,34,35,36]. In previous studies from our center, we reported that liver resection among patients with HCV-related HCC is associated with high incidence of perioperative morbidities. We also, reported a higher incidence of PHLF among Egyptian patients with HCV-related HCC compared to other studies [5, 8].
Tumor recurrence after liver resection for HCC negatively affects the prognosis of HCC patients. Tumor recurrence may occur early after resection owing to multicentric carcinogenesis or late after resection because of the presence of coexisting background of liver cirrhosis [37]. Several factors had been identified to affect tumor recurrence which could be classified to tumor-related, procedure-related, and patient-related factors [38,39,40]. Lurje et al. in a study evaluating tumor recurrence and patients’ survival after curative liver resection for HCC addressed that tumors within Milan criteria, macrovascular invasion, and tumor stage were independently associated with recurrence, while macrovascular invasion and MELD score were independently associated with survival [40]. Wang et al. in a study evaluating the long-term outcomes after liver resection for HCC addressed that in patients with Ishak stage 1 to 5, tumor size was associated with postoperative mortality, and tumor size, and AFP > 20 ng/ml were associated with recurrence rate. On the other hand, poorly differentiated histology and tumor size were associated with higher mortality, and tumor size was associated with recurrence in patients with Ishak stage 6 [41]. Dai et al. in a study evaluating the impact of tumor size on the prognosis of HCC in patients who underwent liver resection showed the important role of tumor size and advanced fibrosis in predicting postoperative mortality and the role of tumor size and histopathological differentiation in predicting HCC recurrence [42]. In the current study, the survival outcomes of the study patients were clearly stratified according to the tumor size. Patients with smaller tumors showed better survival outcomes while patients with larger tumors experienced worser survival outcomes. There were significant differences between the groups regarding the overall survival but not the disease-free survival.
The current study has some limitations. This is a retrospective, single-center study which is liable to selection bias. Secondly, we included patients with HCCs on top of hepatitis C virus, which is the commonest risk factor among Egyptian patients. On the other hand, we did not include any modality of artificial intelligence or machine learning in the current analysis which is one of the most critical advancements in the recent years and is growing in popularity for analysis of large amounts of data. A future multicenter prospective study is needed to validate our results.
Conclusion
In conclusion, the current study suggested that the tumor size is not a contraindication for liver resection. Patients with larger tumors showed higher incidence of perioperative morbidities, especially PHLF and respiratory complications, but comparable peroperative mortality. Patients with larger tumors showed comparable recurrence free survival but poorer overall survival when compared to their counters with smaller tumors.
Availability of the data and materials
The data generated and analyzed for the current manuscript is not publicly available and will be available by a reasonable request from the corresponding author.
References
Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–91.
Ikeda K. Recent advances in medical management of hepatocellular carcinoma. Hepatol Res. 2019;49(1):14–32.
McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73:4–13.
Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015;19(7):1281–90.
Shehta A, Farouk A, Fouad A, Aboelenin A, Elghawalby AN, Said R, et al. Post-hepatectomy liver failure after hepatic resection for hepatocellular carcinoma: a single center experience. Langenbecks Arch Surg. 2021;406(1):87–98.
Santambrogio R, Barabino M, D’Alessandro V, Iacob G, Opocher E, Gemma M, Zappa MA. Microinvasive behaviour of single small hepatocellular carcinoma: which treatment? Updat Surg. 2021;73:1359–69.
Lerut J, Foguenne M, Lai Q. Hepatocellular cancer selection systems and liver transplantation: from the tower of babel to an ideal comprehensive score. Updat Surg. 2021;73(5):1599–614.
Wahab MA, Shehta A, Hamed H, El Nakeeb A, Salah T. Predictors of recurrence in hepatitis C virus related hepatocellular carcinoma after hepatic resection: a retrospective cohort study. The Eurasian journal of medicine. 2014;46(1):36.
Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502–10.
Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery. 2000;127:603–8.
Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–6.
Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–36.
Zhang H, Yuan SX, Dai SY, Zhang JM, Huang X, Lu CD, et al. Tumor size does not independently affect long-term survival after curative resection of solitary hepatocellular carcinoma without macroscopic vascular invasion. World J Surg. 2014;38:947–57.
Shehta A, Farouk A, Elghawalby AN, Elshobary M, Aboelenin A, Fouad A, et al. Outcomes of hepatic resection for hepatocellular carcinoma associated with portal vein invasion. J Surg Res. 2021;266:269–83.
Wolters U, Wolf T, Stutzer H, Schroder T. ASA classification and perioperative variables as predictors of postoperative outcomes. Br J Anaesth. 1996;77:217–22.
Terminology Committee of the International Hepato-Pancreato- Biliary Association. The IHPBA Brisbane 2000 terminology of liver anatomy and resections. HPB Surg. 2000;2:333–9.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–24.
Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–8.
Rahbari NN, Garden OJ, Padbury R, Maddern G, Koch M, Hugh TJ, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB. 2011;13(8):528–35.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–86.
Waked I, Esmat G, Elsharkawy A, El-Serafy M, Abdel-Razek W, Ghalab R, et al. Screening and treatment program to eliminate hepatitis C in Egypt. N Engl J Med. 2020;382:1166–74.
Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005;11:7391–400.
Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91.
Chung H, Kudo M, Takahashi S, Hagiwara S, Sakaguchi Y, Inoue T, et al. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol. 2008;23(3):445–52.
Blajchman MA. Immunomodulation and blood transfusion. Am J Ther. 2002;9:389–95.
Krensky AM, Clayberger C. Structure of HLA molecules and immune-suppressive effects of HLA derived peptides. Int Rev Immunol. 1996;13:173–85.
Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7.
Llovet JM, Bustamante J, Castells A, Vilana R, del C Ayuso M, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7.
Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938–43.
Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: Is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East-West study group. Ann Surg. 2013;257:929–37.
Higaki T, Yamazaki S, Moriguchi M, Nakayama H, Kurokawa T, Takayama T. Indication for surgical resection in patients with hepatocellular carcinoma with major vascular invasion. Bioscience trends. 2017;11(5):581–7.
Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118:4725–36.
Liu PH, Lee YH, Hsia CY, Hsu CY, Huang YH, Chiou YY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014;21:1825–33.
Taura K, Ikai I, Hatano E, Yasuchika K, Nakajima A, Tada M, et al. Influence of coexisting cirrhosis on outcomes after partial hepatic resection for hepatocellular carcinoma fulfilling the Milan criteria: an analysis of 293 patients. Surgery. 2007;142(5):685–94.
Poon RTP, Fan ST, Ng IOL, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–7.
Pinto A, Hobeika C, Philis A, Kirzin S, Carrère N, Ghouti L. Synchronous liver metastases and peritoneal carcinomatosis from colorectal cancer: different strategies for curative treatment? Langenbeck’s Arch Surg. 2019;404(4):477–88.
Lurje G, Bednarsch J, Czigany Z, Amygdalos I, Meister F, Schöning W, et al. Prognostic factors of disease-free and overall survival in patients with hepatocellular carcinoma undergoing partial hepatectomy in curative intent. Langenbeck’s Arch Surg. 2018;403(7):851–61.
Wang Q, Fiel MI, Blank S, Luan W, Kadri H, Kim KW, et al. Impact of liver fibrosis on prognosis following liver resection for hepatitis B-associated hepatocellular carcinoma. Br J Cancer. 2013;109:573–81.
Dai CY, Lin CY, Tsai PC, Lin PY, Yeh ML, Huang CF, et al. Impact of tumor size on the prognosis of hepatocellular carcinoma in patients who underwent liver resection. J Chin Med Assoc. 2018;81(2):155–63.
Acknowledgements
None.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No external funding resources.
Author information
Authors and Affiliations
Contributions
Conception and design of the manuscript: Ahmed Shehta, Ahmed Farouk. Acquisition of data: Mohamed Medhat, Ahmed Farouk, Ahmed Shehta. Analysis and interpretation of data: Ahmed Shehta. Drafting the manuscript: All authors. Critical revision of the final version to be published: All authors. Approval of the final version to be published: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An informed consent was obtained from each patient prior to surgical intervention. The study was approved by the Institutional Review Board and Local Ethical Committee at the Faculty of Medicine, Mansoura University, Egypt.
Consent of publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shehta, A., Elsabbagh, A.M., Medhat, M. et al. Impact of tumor size on the outcomes of hepatic resection for hepatocellular carcinoma: a retrospective study. BMC Surg 24, 7 (2024). https://doi.org/10.1186/s12893-023-02296-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-023-02296-w