Abstract
Introduction
The susceptibility to surgical site occurrence (SSO) is high following ventral hernia repair (VHR) surgery. SSO severely increases the physical and mental burden on patients. The main purpose of this review was to analyze the efficacy of negative pressure wound therapy (NPWT) after open VHR(OVHR) and explore benefits to patients.
Methods
The Cochrane Library, PubMed, and Embase databases were searched from the date of establishment to 15 October 2022. All randomized controlled trials and retrospective cohort studies comparing NPWT with standard dressings after OVHR were included. The Revman 5.4 software recommended by Cochrane and the STATA16 software were used in this meta-analysis.
Results
Fifteen studies (involving 1666 patients) were identified and included in the meta-analysis, with 821 patients receiving NPWT. Overall, the incidence rate of SSO in the NPWT group was lower compared to the control group (odds ratio [OR] = 0.44; 95% confidence interval [CI] = 0.21–0.93; I2 = 86%; P = 0.03). The occurrence rate of surgical site infection (SSI; OR = 0.51; 95% CI = 0.38–0.68, P < 0.001), wound dehiscence (OR = 0.64; 95% CI = 0. 43–0.96; P = 0.03), and hernia recurrence (OR = 0.51; 95% CI = 0.28–0.91, P = 0.02) was also lowered. There was no significant difference in seroma (OR = 0.76; 95% CI = 0.54–1.06; P = 0.11), hematoma (OR = 0.53; 95% CI = 0.25–1.11; P = 0.09), or skin necrosis (OR = 0.83; 95% CI = 0.47–1.46; P = 0.52).
Conclusion
NPWT can effectively decrease the occurrence of SSO, SSI wound dehiscence and hernia recurrence and should be considered following OVHR.
Similar content being viewed by others
Introduction
Ventral hernia (VH) is a common complication after abdominal surgery. About 10 to 23% of patients develop incisional hernias [1]. The United States of America (USA) spends more than $3.2 billion per year on surgical treatment of adult ventral hernia [2]. Compared to low body mass index (BMI), patients with obesity have a higher incidence of abdominal wall hernias, larger hernia defects, poorer prognosis, and a higher risk of hernia recurrence. Open ventral hernia repair (OVHR) is favorable for large and complex anatomy due to advantages, such as clear anatomy, low operation difficulty, low effect of obesity, short anesthesia time, and low impact on intestinal function [3]. However, the potential dead space and mesh after OVHR can easily lead to surgical site occurrence (SSO), including surgical site infection (SSI), wound dehiscence, seroma, hematoma, skin necrosis, and hernia recurrence [4]. SSIs and other complications may further lead to extended hospital stays, delayed recovery, increased psychological stress, and higher treatment costs [5]. Some factors associated with SSI include weakened immunity, poor nutrition, advanced age, diabetes, use of corticosteroids, and incarcerated hernia. Therefore, breakthrough techniques in surgery are a priority for patients with high-risk factors for SSI.
Negative pressure wound therapy (NPWT) is a widely used wound care technology, mainly for soft tissue trauma and fracture [6]. NPWT includes a drainage system involving the wound dressing and its connected device. By applying negative pressure ranging from − 75 mmHg to − 150 mmHg, any wound and tissue fluid drawn from the area is collected into the vacuum device [7]. Negative pressure suction devices can be used in several ways. Their advantages include removing wound exudates, promoting apposition of the skin edges, changing the microenvironment and stimulating the formation of granulation tissue, and new blood vessel formation [8]. NPWT has been shown to reduce the incidence of complications in various primary closures [9]. However, the efficacy of NPWT after OVHR is unclear. The main purpose of our systematic review is to highlight the effect of prophylactic negative pressure wound therapy (pNPWT) in preventing SSO. The secondary outcome was the incidence rate of other complications.
Methods
Data strategy
The Cochrane Central Register of Controlled Trials, MEDLINE, and Embase databases were searched from the respective dates of inception until 15 October 2022. A combination of Mesh and text terms, such as “ventral hernia repair,” “incisional hernia repair,” “VHR,” “abdominal wall reconstruction,” “negative pressure therapy,” “negative pressure wound therapy,” “NPWT,” “VAC,” and “vacuum-assisted closure,” were used. References from related articles, reviews, and meta-analyses were manually searched. This review conformed to the AMSTAR checklist and adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [10].
Inclusion criteria
This review mainly included randomized controlled trials (RCTs) and retrospective cohort studies comparing pNPWT with standard dressing after OVHR. Only articles with adult patients (> 18 years of age) undergoing OVHR were included in the analysis. The intervention group included pNPWT technique using devices such as PREVENA, PICO, and VAC based on different degrees of negative pressure.
Exclusion criteria
The excluded articles were those with pNPWT used alone or compared with other technologies; reporting no outcomes; systematic reviews, study protocols, and case reports on the placement of pNPWT in inguinal and perineal hernias; and published in a language other than English.
Outcomes
SSO was the primary outcome of this review. SSI, seroma, hematoma, wound dehiscence, skin necrosis, and hernia recurrence were the secondary outcomes considered to further analyze the effect on SSO results.
Data collection and analysis
Two authors independently examined the title and abstract of each article after electronic retrieval. In case of disagreements, a third independent author evaluated the article. The articles that potentially met the inclusion criteria were further analyzed, and only those meeting all inclusion criteria were used for analysis.
Quality assessment of included article
Cochrane tools were used to assess the RCT bias, including selection bias, performance bias, detection bias, attrition bias, reporting bias and other sources of bias. The Newcastle-Ottawa Cohort Study Quality Assessment Scale was used to assess the risk of cohort study bias. A third independent author decided inclusion in case of disagreements between the two authors.
Statistical analysis
All statistical analyses were performed using the STATA16 software and Cochrane’s Review Manager 5.4 software. Meta-analysis was performed on the odds ratio (OR) of the binary variables and a P-value of < 0.05 was considered statistically significant. The I2 statistic was used to assess intergroup heterogeneity. The random-effects model and fixed-effects model were used, according to the heterogeneity among included studies. The results of the meta-analysis were presented graphically as forest plots. Egger’s test was used to assess publication bias according to the number of articles.
Results
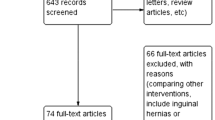
Overall, a total of 476 studies were identified from the databases. Fifty full texts were then shortlisted based on title and abstract analysis, of which 15 articles finally met the inclusion criteria. The specific inclusion process flowchart is shown in Fig. 1. A total of 1666 patients were included in the selected articles, of which 821 patients underwent pNPWT.
The general characteristics of the articles included in the meta-analysis are shown in Table 1. The sample size of these studies ranged from 35 to 180 and the maximum follow-up duration varied from 30 days to 38.5 months. Most of the studies were retrospective cohort analyses and only 2 were RCTs. PREVENA was the most widely used pNPWT device for OVHR. PREVENA was used and described in eight articles. The negative pressure applied to the incision varied from − 75 mmHg to − 125 mmHg.
Assessment of the included studies
According to the Newcastle-Ottawa Scale for retrospective cohort study, 9 cohort studies were ranked as high quality (scores≥7) and 4 studies as medium quality (Table. 2). Only Bueno-Lledó and Mondal conducted RCTs, both of which had detailed descriptions of randomization and data collection except blinding (Fig. 2).
SSO
In the SSO analysis, a total of 8 articles were included and 1208 patients were involved. A random-effects model was used in the analysis considering the high heterogeneity (I2 = 86%, P < 0.001). The rate of SSO was significantly different in patients who received pNPWT compared with those who received standard dressing. About 24.6% (159/646) of patients who underwent pNPWT reported SSO at follow-up compared to 40.0% (225/562) who received standard dressings (pooled OR = 0.44; 95% confidence interval [CI] = 0.21–0.93; P = 0.03; Fig. 3). Subgroup analysis revealed differences in dressings and article quality as possible sources of heterogeneity (P ≤ 0.05). Due to the low number of studies, Egger’s test was used to evaluate publication bias and the results revealed no definite publication bias (P > 0.05).
SSI
After pooling all included studies reporting SSI rates (14 studies, 1486 patients), we found that 11.4% (76 of 665) of the patients who received pNPWT developed SSI compared with 14.0% (105 of 751) of those who received standard dressing; pNPWT could effectively reduce the SSI rate (pooled OR = 0.51; 95% CI = 0.38–0.68; P < 0.001; Fig. 4). Medium heterogeneity in this analysis (I2 = 45%) and the random effects model were selected.
Seroma and hematoma
Seroma and hematoma were used as outcome measures in 14 and 9 studies, respectively. The fixed-effects model was used for the results because none suggested high heterogeneity (seroma: I2 = 24%; hematoma: I2 = 0%; Figs. 5 and 6). There was no significant difference between the pNPWT group and control group (standard dressing) in seroma (OR = 0.76; 95% CI = 0.54–1.06; P = 0.11) and hematoma (OR = 0.53; 95% CI = 0.25–1.11; P = 0.09).
Wound dehiscence and skin necrosis
Eleven and 5 studies included wound dehiscence and skin necrosis as outcome measures, respectively. Heterogeneity was ascertained as low (wound dehiscence: I2 = 23%; skin necrosis: I2 = 14%; Fig. 7 and Fig. 8). Prophylactic NPWT appeared to be protective against wound dehiscence compared to standard dressing (OR = 0.64; 95% CI = 0. 43–0.96; P = 0.03). The occurrence of skin necrosis was evident in 7.3% (28/359) of patients after pNPWT compared to 9.2% (27/294) of patients after standard dressing (P = 0.52).
Hernia recurrence
Seven studies included hernia recurrence rate as an outcome measure. A fixed-effect model was used in the analysis considering low heterogeneity (I2 = 24%, P = 0.25). Patients receiving pNPWT had a significantly decreased risk of hernia recurrence compared with standard dressing; 4.7% (21/449) of patients who received pNPWT reported SSO at follow-up compared to 8.0% (32/401) of those who received standard dressings (pooled OR = 0.51; 95% CI = 0.28–0.91; P = 0.02; Fig. 9).
Discussion
VHR is a common surgical procedure, with more than 400,000 surgeries performed in the USA alone annually [26]. Laparoscopic VHR is becoming a general trend due to its lower perioperative complication rate, shorter hospital stays, and lower postoperative readmission rate [27]. Regardless of the surgical method (whether open or endoscopic), postoperative complications remain a challenge for surgeons. Without a doubt, the outcomes could be extremely complex, leading to increased medical costs to patients and hospitals.
NPWT is commonly used to treat open wounds in the abdomen in combination with lavage, debridement, and bowel resection as indicated [28]. Since its introduction in 1997, NPWT has been shown to be effective in both open and postoperative closed wounds [29]. However, the efficacy of pNPWT in wound healing after OVHR is unclear and data are contradictory in the literature [30]. In the earliest RCT in 2020 including 150 patients, Bueno-Lledó et al. showed that the incidence of SSO in the control group (standard dressing; 22/74, 29.8%) was significantly higher than that in the NPWT group (12/72, 16.6%, P < 0.05) after 30 days of postoperative follow-up [31]. Deldar et al. found that NPWT had no effect on the prevention of SSO (23.2% vs 26.3%, P = 0.663) [23]. In addition, the two RCTs to date have different results in the SSI study [11]. The existing related studies were mostly retrospective cohort studies or case series. We analyzed 15 articles and the results of the meta-analysis from all included studies showed that pNPWT significantly reduced the risk of SSO, SSI, wound dehiscence, and the rate of hernia recurrence (P < 0.05). Meanwhile, there was no significant difference in seroma, hematoma, and skin necrosis with pNPWT compared to standard wound dressings.
It is worth noting that with the addition of a recent RCT, we obtained the same results for SSO as in the previously published meta-analysis articles [32]. Nevertheless, a high degree of heterogeneity (I2 = 87%) is seen in the SSO results. Further subgroup analysis suggested that the heterogeneity stemmed from differences in dressings and the quality of studies. The probable reason for the difference in the test results is variations in the diagnostic methods and surgeon-dependent definition of SSO and operation level. However, due to the small number of studies, we did not use the traditional funnel plot for analysis of publication bias. Also, Egger’s test results revealed no publication bias in SSO. No high heterogeneity was found in the analysis of SSI, seroma, hematoma, wound dehiscence, skin necrosis, and hernia recurrence.
NPWT has been shown to have a crucial role in scar healing following OVHR [33]. NPWT can enhance wound drainage, eliminate bacterial byproducts, discharge secretions efficiently, and clear necrotic tissue [34]. Under the mechanical traction of negative pressure, the differential pressure between the inner and outer capillaries and the endothelial cell space of lymphatic capillaries increases, resulting in elevated levels of blood supply and lymphatic reflux [35,36,37]. Compared with conventional dressings, a negative pressure provides effective and sustained support for local wound circulation, thereby reducing the risk of seroma and hematoma [11, 38]. Furthermore, continuous negative pressure extracts interstitial fluid from the wound, fosters growth of capillaries and granulation tissue, improves blood circulation, and accelerates scar healing [39, 40]. Through these mechanisms, NPWT lowers wound healing time and enhances scar recovery significantly. Additional studies are required to further understand the mechanism of NPWT in OVHR.
However, NPWT may entail certain complications, such as skin blisters [33], which typically resolve spontaneously within approximately a week [41]. Improper usage of NPWT has the potential to result in more severe complications, including skin necrosis, bleeding, and allergic reactions [42, 43]. It is imperative to discontinue NPWT in case these complications occur. Nevertheless, studies indicate that there is no noteworthy increase in the incidence of wound-related adverse events associated with NPWT when compared to standard dressings [44]. Appropriate preventive measures may help mitigate adverse events [45].
There are some limitations of this meta-analysis. First, most studies involved in this meta-analysis were retrospective and only two RCTs were included, which may introduce a selection bias in the process [46]. Second, the definition of SSO was different among articles. Most authors defined SSO as surgical site infection (SSI), wound dehiscence, skin necrosis, seroma, and hematoma. Seaman et al. believed that enterocutaneous fistula, mesh infection, hernia recurrence, and/or 30-day bulge are considered when detecting SSO [30], which is bound to make a difference to the SSO results. Third, there is a lack of standard diagnostic criteria and follow-up time for complications occurring in these studies. For example, seroma was diagnosed clinically or using ultrasound, with significantly different incidences. Further, the shortest follow-up period was 1 month, while the longest follow-up period was 38.5 months. Fourth, most of the articles did not report any recurrent hernias or emergency hernias, which might also affect the occurrence of SSO. Finally, there was no standard for the defect range in the included articles. Only a few studies used the defect size or European Hernia Society (EHS) classification. The guidelines of the International Endohernia Society (IEHS) also clearly state that abdominal wall defects are closely related to postoperative SSO and the recurrence of hernia [47]. This also requires newer studies to further verify how large a defect is suitable for placing pNPWT.
Certainly, if only the device cost is considered, the cost of closing surgical wounds with NPWT is higher than that with traditional dressings. In the study by Clare et al., after accounting for postoperative complications and hospitalization costs, a cost-benefit analysis showed an estimated total cost reduction of €170,944.00 in 100 people treated with closed incision negative pressure therapy (ciNPT) compared to standard wound care [15]. Svensson-Björk et al. concluded that NPWT can reduce the incidence of SSI, which was more cost-effective than standard dressings; the average cost increase was €1853 after following patients for 90 days following open inguinal vascular surgery [48]. Considering the cost of analgesics, antibiotics, hospitalization, and morbidity, Condon et al. concluded that the cost of satisfactory healing in people who received NPWT was lower compared to conventional dressing for the treatment of wounds due to diabetic foot [19].
Conclusions
Our review concludes that the use of pNPWT after OVHR can reduce the incidence of SSO and SSI, wound dehiscence, and hernia recurrence simultaneously. Prophylactic NPWT has no obvious effect on other complications, such as seroma, hematoma, and skin necrosis. However, the large heterogeneity of studies regarding SSO is a limitation of the review, possibly due to differences in article quality and NPWT techniques. There is an urgent need for higher quality, better-designed RCTs with consistent standards to further verify the efficacy of pNPWT following OVHR.
Availability of data and materials
All authors have agreed to make provisions for all materials described in this manuscript including relevant raw data to be freely available to any researchers.
Abbreviations
- VHR:
-
Ventral hernia repair
- SSO:
-
Surgical site occurrence
- NPWT:
-
Negative pressure wound therapy
- OVHR:
-
Open ventral hernia repair
- RCTs:
-
Randomized controlled trials
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SSI:
-
Surgical site infection
- VH:
-
Ventral hernia
- BMI:
-
Body mass index
- pNPWT:
-
Prophylactic negative pressure wound therapy
- VAC:
-
Vacuum-assisted closure
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- EHS:
-
European Hernia Society
- IEHS:
-
International Endohernia Society
- ciNPT:
-
Closed incision negative pressure therapy
References
Albino FP, Patel KM, Nahabedian MY, Attinger CE, Bhanot P. Immediate, multistaged approach to infected synthetic mesh: outcomes after abdominal wall reconstruction with porcine acellular dermal matrix. Ann Plast Surg. 2015;75(6):629–33.
Holihan JL, Hannon C, Goodenough C, Flores-Gonzalez JR, Itani KM, Olavarria O, Mo J, Ko TC, Kao LS, Liang MK. Ventral hernia repair: a meta-analysis of randomized controlled trials. Surg Infect. 2017;18(6):647–58.
Liang MK, Holihan JL, Itani K. Ventral hernia management: expert consensus guided by systematic review. Ann Surg. 2016;.
Peterman DE, Warren JA. Ventral hernia management in obese patients. Surg Clinics. 2021;101(2):307–21.
Gabriel A, Gupta S, Orgill DP. Challenges and management of surgical site occurrences. Plast Reconstr Surg. 2019;143(1S):7S–10S.
Broex EC, Van Asselt AD, Bruggeman CA, Van Tiel FH. Surgical site infections: how high are the costs? J Hosp Infect. 2009;72(3):193–201.
Iheozor‐Ejiofor Z, Newton K, Dumville JC, Costa ML, Norman G, Bruce J. Negative pressure wound therapy for open traumatic wounds. Cochrane Database Syst Rev. 2018;7.
Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38(6):563–77.
Peinemann F, Sauerland S. Negative-pressure wound therapy: systematic review of randomized controlled trials. Dtsch Arztebl Int. 2011;108(22):381.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W–65.
Bueno-Lledo J, Franco-Bernal A, Garcia-Voz-Mediano MT, Torregrosa-Gallud A, Bonafe S. Prophylactic single-use negative pressure dressing in closed surgical wounds after incisional hernia repair: a randomized. Controlled Trial Ann Surg. 2021;273(6):1081–6.
Conde-Green A, Chung TL, Holton LH 3rd, Hui-Chou HG, Zhu Y, Wang H, et al. Incisional negative-pressure wound therapy versus conventional dressings following abdominal wall reconstruction: a comparative study. Ann Plast Surg. 2013;71(4):394–7.
de Vries FEE, Atema JJ, Lapid O, Obdeijn MC, Boermeester MA. Closed incision prophylactic negative pressure wound therapy in patients undergoing major complex abdominal wall repair. Hernia. 2017;21(4):583–9.
Deldar R, Abu El Hawa AA, Bovill JD, Hipolito D, Tefera E, Bhanot P, et al. Negative pressure wound therapy prevents hernia recurrence in simultaneous ventral hernia repair and Panniculectomy. Plast Reconstr Surg Glob Open. 2022;10(3):e4171.
Diaconu SC, McNichols CHL, Ngaage LM, Liang Y, Ikheloa E, Bai J, et al. Closed-incision negative-pressure therapy decreases complications in ventral hernia repair with concurrent panniculectomy. Hernia. 2020 Feb;24:49–55.
Gassman A, Mehta A, Bucholdz E, Abthani A, Guerra O, Maclin MM Jr, et al. Positive outcomes with negative pressure therapy over primarily closed large abdominal wall reconstruction reduces surgical site infection rates. Hernia. 2015;19(2):273–8.
Hopkins B, Eustache J, Ganescu O, Cipolla J, Kaneva P, Fried GM, et al. S116: impact of incisional negative pressure wound therapy on surgical site infection after complex incisional hernia repair: a retrospective matched cohort study. Surg Endosc. 2021;35(7):3949–60.
Leuchter M, Hitzbleck M, Schafmayer C, Philipp M. Use of incisional preventive negative pressure wound therapy in open incisional hernia repair: who benefits? Wound Repair Regen. 2021;29(5):759–65.
Licari L, Campanella S, Carolla C, Viola S, Salamone G. Closed incision negative pressure therapy achieves better outcome than standard wound care: clinical outcome and cost-effectiveness analysis in open ventral hernia repair with synthetic mesh positioning. Cureus. 2020;12(5).
Mondal A, Ali M, Galidevara I, Arumugam M. Effect of incisional negative pressure wound therapy following incisional hernia RepairA randomised controlled trial. J Clin Diagn Res. 2022;16.
Olona C, Duque E, Caro A, Jiménez A, Moreno F, Coronas JM, et al. Negative-pressure therapy in the postoperative treatment of incisional hernioplasty wounds: a pilot study. Adv Skin Wound Care. 2014;27(2):77–80.
Pauli EM, Krpata DM, Novitsky YW, Rosen MJ. Negative pressure therapy for high-risk abdominal wall reconstruction incisions. Surg Infect. 2013;14(3):270–4.
Seaman AA-OX, Sarac BA-O, ElHawary HA-O, Janis JA-O. The effect of negative pressure wound therapy on surgical site occurrences in closed incision abdominal wall reconstructions: a retrospective single surgeon and institution study. Hernia. 2021;25(6):1549–55.
Soares KC, Baltodano PA, Hicks CW, Cooney CM, Olorundare IO, Cornell P, et al. Novel wound management system reduction of surgical site morbidity after ventral hernia repairs: a critical analysis. Am J Surg. 2015;209(2):324–32.
Wang E, Archer L, Foster A, Ballal M. Large abdominal hernia repair with closed incision negative pressure therapy: a case series. J Wound Care. 2021;30(3):192–6.
Norman G, Shi C, Goh EL, Murphy EM, Reid A, Chiverton L, Stankiewicz M, Dumville JC. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2022;4.
Webster J, Liu ZM, Norman G, Dumville JC, Chiverton L, Scuffham P, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2019(3).
Orgill DP, Bayer LR. Update on negative-pressure wound therapy. Plast Reconstr Surg. 2011;127:105S–15S.
Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D, Beck W, Holzman MD. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia. 2012;16:179–83.
Ecker BL, Kuo LE, Simmons KD, Fischer JP, Morris JB, Kelz RR. Laparoscopic versus open ventral hernia repair: longitudinal outcomes and cost analysis using statewide claims data. Surg Endosc. 2016;30:906–15.
Heniford BT, Ramshaw BJ. Laparoscopic ventral hernia repair: a report of 100 consecutive cases. Surg Endosc. 2000;14:419–23.
Deldar R, Abu El Hawa AA, Bovill JD, Hipolito D, Tefera E, Bhanot P, et al. Negative Pressure Wound Therapy Prevents Hernia Recurrence in Simultaneous Ventral Hernia Repair and Panniculectomy. Plastic Reconst Surg Global Open. 10(3).
Qiu XT, Luo HM, Huang GB. Roles of negative pressure wound therapy for scar revision. Front Physiol. 2023;14.
Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: A review. The Spine J. 13(10):1393–405.
Huang Q, Huang K, Xue J. Vacuum sealing drainage combined with free anterolateral femoral skin flap grafting in 16 cases of pediatric soft tissue damage to the foot and ankle. Transl Pediat. 10(10):2489.
Yang H, Liu L, Li G, Chen Y, Jiang D, Wang W, et al. Growth promoting effect of vacuum sealing drainage in the healing processes of diabetic foot ulcers. Therapeut Clin Risk Manag. :65–71.
Cagney DA-O, Simmons L, O'Leary DP, Corrigan M, Kelly L, O'Sullivan MJ, et al. The efficacy of prophylactic negative pressure wound therapy for closed incisions in breast surgery: a systematic review and meta-analysis. Surg Res Practice. 2022;.
Yang SL, Zhu LY, Han R, Sun LL, Dou JT. Effect of Negative Pressure Wound Therapy on Cellular Fibronectin and Transforming Growth Factor-β1 Expression in Diabetic Foot Wounds. Foot Ankle Int. 2017;38(8):893–900.
Zwanenburg PR, Tol BT, Obdeijn MC, Lapid O, Gans SL, Boermeester MA. Meta-analysis, meta-regression, and grade assessment of randomized and nonrandomized studies of incisional negative pressure wound therapy versus control dressings for the prevention of postoperative wound complications. Ann Surg. 2020;272(1):81–91.
Sun T, Ying W, Wang S, Chen C, Sun P, Tan J. Clinical application of vacuum sealing drainage for the treatment of deep burn wounds. The Am Surg™. 2023;89(4):1018–23.
Kuo FC, Hsu CW, Tan TL, Lin PY, Tu YK, Chen PC. Effectiveness of different wound dressings in the reduction of blisters and periprosthetic joint infection after total joint arthroplasty: a systematic review and network meta-analysis. The J Arthroplast. 2021;36(7):2612–29.
Ji S, Liu X, Huang J, Bao J, Chen Z, Han C, et al. Consensus on the application of negative pressure wound therapy of diabetic foot wounds. Burns & Trauma. 2021;9:tkab018.
Zhang C, Wang Q, Wang Z, Huang Q, Zhang C, Duan N, et al. Modified negative-pressure wound therapy for linear blister formation prevention around foam dressings: technical note and case series. J Orthop Surg Res. 2021;16(1):1–8.
Notorgiacomo G, Klug J, Rapp S, Boyce ST, Schutte SA-O. A bioreactor for studying negative pressure wound therapy on skin grafts. Int Wound J. 2022;19(3):633–42.
Wilkinson HA-O, Longhorne FL, Roberts ER, Brownhill VR, Hardman MA-O. Cellular benefits of single-use negative pressure wound therapy demonstrated in a novel ex vivo human skin wound model. Wound Repair Regen. 2021;29(2):298–305.
Mondal A, Ali MS, Galidevara I, Arumugam M. Effect of incisional negative pressure wound therapy following incisional hernia repair-a randomised controlled trial. J Clin Diagn Res. 2022;16(2):PC01–PC4.
Berner-Hansen VA-O, Oma EA-O, Willaume MA-O, Jensen KA-O. Prophylactic negative pressure wound therapy after open ventral hernia repair: a systematic review and meta-analysis. Hernia. 2021 Dec;25(6):1481–90.
Bittner R, Bain K, Bansal VK, Berrevoet F, Bingener-Casey J, Chen D, et al. Update of Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS))-Part A. Surg Endosc. 2019;33:3069–139.
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Funding
This work was supported by the “1 + X” program for the Clinical Competency Enhancement - Interdisciplinary Innovation Project, The Second Hospital of Dalian Medical University (2022JCXKYB01, 2022JCXKYB20), and the Dalian Medical Science Research Project (2012023).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yang Xu, Shuai Shao, and Xin Chen; investigation: ZeZhong Gong, ZhaoHui Xu, HaoNan Kang, HyokJu Ri, and Boureima Hamidou Amadou; data curation: Yan Shan, YanYing Ren, and Fan Zhang; writing-original and original draft preparation: Yang Xu, HaoNan Kang, and Xin Chen; writing, reviewing and editing: ZhaoHui Xu, ZeZhong Gong, Yan Shan, YanYing Ren, and Fan Zhang; and supervision and project administration: HyokJu Ri, Xin Chen, and Boureima Hamidou Amadou. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was not required for this article.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, Y., Shao, S., Gong, Z. et al. Efficacy of prophylactic negative pressure wound therapy after open ventral hernia repair: a systematic review meta-analysis. BMC Surg 23, 374 (2023). https://doi.org/10.1186/s12893-023-02280-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-023-02280-4