Abstract
Background
Esophagectomy is the gold-standard treatment for locally advanced esophageal cancer but has high morbimortality rates. Sarcopenia is a common comorbidity in cancer patients. The exact burden of sarcopenia in esophagectomy outcomes remains unclear. Therefore, this systematic review and meta-analysis were performed to establish the impact of sarcopenia on postoperative outcomes of esophagectomy for cancer.
Methods
We performed a systematic review and meta-analysis comparing sarcopenic with non-sarcopenic patients before esophagectomy for cancer (Registration number: CRD42021270332). An electronic search was conducted on Embase, PubMed, Cochrane, and LILACS, alongside a manual search of the references. The inclusion criteria were cohorts, case series, and clinical trials; adult patients; studies evaluating patients with sarcopenia undergoing esophagectomy or gastroesophagectomy for cancer; and studies that analyze relevant outcomes. The exclusion criteria were letters, editorials, congress abstracts, case reports, reviews, cross-sectional studies, patients undergoing surgery for benign conditions, and animal studies. The meta-analysis was synthesized with forest plots.
Results
The meta-analysis included 40 studies. Sarcopenia was significantly associated with increased postoperative complications (RD: 0.08; 95% CI: 0.02 to 0.14), severe complications (RD: 0.11; 95% CI: 0.04 to 0.19), and pneumonia (RD: 0.13; 95% CI: 0.09 to 0.18). Patients with sarcopenia had a lower probability of survival at a 3-year follow-up (RD: -0.16; 95% CI: -0.23 to -0.10).
Conclusion
Preoperative sarcopenia imposes a higher risk for overall complications and severe complications. Besides, patients with sarcopenia had a lower chance of long-term survival.
Similar content being viewed by others
Background
Esophagectomy is a major surgical procedure with an inherently high risk for postoperative complications [1]. The main complications are anastomotic leak, infection, paralysis of the vocal cords, pulmonary-related complications, and others [2, 3]. The postoperative mortality risk is around 5% [4]. Consequently, a rigorous preoperative risk surgical assessment is necessary to improve postoperative outcomes. In this setting, eligibility for the surgery depends on the patient’s general conditions, including caloric-protein nutritional status [5, 6].
Patients with esophageal cancer often present a malnutrition status. Esophageal cancer leads to obstructive symptoms, as the tumor mass prevents food passage and thus makes it impossible for the patient to intake the necessary calories and nutrients [7]. In addition, the metabolic and physical effects of cancer, with a chronic inflammatory state and excessive catabolism, as well as the side effects of anti-cancer treatments, contribute to cachexia and weight loss [8, 9].
Sarcopenia is a syndrome characterized by loss of strength and skeletal muscle mass [10]. The prevalence of preoperative sarcopenia in patients with esophageal cancer ranges from 14.4 to 80% [2]. The calculation of skeletal muscle mass (SMM), based on the skeletal muscle index (SMI) obtained by computed tomography of the transverse muscle mass at the level of the lumbar vertebras, is the gold standard test to diagnose sarcopenia [11]. Computed tomography is routinely ordered as a preoperative exam for esophageal cancer patients, and consequently, SMM is a promptly accessible and cheap test to investigate sarcopenia [11].
Sarcopenia is related to worse postoperative outcomes due to the increased risk of infection, physical disability, and deficit of tissue regeneration [2, 10]. Consequently, sarcopenia may pose a high risk for patients undergoing esophagectomy [10].
This systematic review and meta-analysis aim to increase the level of evidence with a quantitative synthesis of results that analyze the impact of sarcopenia on postoperative outcomes of patients with esophageal cancer submitted to curative resection.
Methods
The systematic review and meta-analysis was reported and conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) statement [12]. The study protocol was registered on PROSPERO (International Prospective Register of Systematic Reviews) [13] under the registration number CRD42021270332.
Eligibility criteria
The inclusion criteria are cohort studies, case series, and clinical trials; adult patients (> 18 years old); studies evaluating patients with sarcopenia undergoing esophagectomy or gastroesophagectomy for cancer; and studies that analyze relevant outcomes.
The exclusion criteria are letters, editorials, congress abstracts, case reports, reviews, cross-sectional studies, patients undergoing surgery for reasons other than esophageal cancer, and animal studies.
Information sources and search strategy
An online search was conducted in parallel and independently by two reviewers through PubMed, Embase, Cochrane Library Central, and Lilacs (BVS), alongside a manual search of references from all included studies, previous systematic reviews and meta-analyses. The search strategy was developed from the databases’ inception to December 2022 based on a combination of MeSH terms and keywords on Medline and Embase ((esophagectom* OR esophageal resection OR esophag* excision OR esophagus resection OR esophag* removal OR oesophago-gastrectomy OR oesophagectom*) AND (sarcopen* OR muscle loss OR muscle dystrophy OR muscle atrophy OR muscle atrophies OR muscle weakness OR muscle wasting OR muscle degeneration OR muscular loss OR muscular degeneration OR muscular atrophies OR muscular dystrophy OR cachexia OR cachectic) ; Lilacs ((sarcopen* OR muscle loss OR perda muscular OR muscle atroph* OR atrofia muscular OR cachexia) AND (esophagectom* OR oesophago-gastrectomy OR esophageal resection OR oesophagectomy OR (esophageal AND surgical resection)) AND (esophagus tumor OR esophagus cancer OR câncer de esôfago OR malign esophagus)); Cochrane ((esophagectom* OR esophageal resection OR esophageal excision OR esophagus excision OR esophagus removal OR oesophago-gastrectomy) AND (sarcopen* OR muscle loss OR muscle weakness OR muscle wasting OR muscular loss)).
Study selection
Two reviewers conducted the study selection in parallel and independently. In case of conflict concerning the inclusion of a study, a third more experienced reviewer solved it after a group discussion where both parties were taken into consideration. The study selection was initially by title evaluation, abstract, and later by full-text analysis, following the predefined eligibility criteria. No restrictions were applied on either language or period of publication. No filters were used for selection.
Data extraction
The baseline characteristics of the included studies were extracted, such as mean age, sex, esophageal cancer type, clinical staging, neoadjuvant therapy, type of esophagectomy, and the outcomes-related variables, such as postoperative mortality, postoperative complications, anastomotic leak, length of hospital stay, and length of ICU stay.
Statistical analysis and data synthesis
Data were manually extracted independently by two reviewers and then meta-analyzed using the Software STATA 16.0 (StataCorp LLC). The summary results were expressed as risk difference (RD) for categorical variables and mean differences (MD) for continuous variables. A 95% confidence interval was applied. Statistical heterogeneity was evaluated using the I2 test A random effect model was applied to weigh the statistical and clinical heterogeneity. The meta-analysis was synthesized with forest plots.
In addition, a subset of studies that assessed sarcopenia with Skeletal Muscle Mass Index (SMI) was performed to investigate the robustness of the meta-analysis. Both fixed and random effect models were applied for this subset of studies as sensitivity analyses.
Risk of bias assessment
All eligible studies considered went through the risk of bias assessment by the Newcastle Ottawa scale [14], a tool typically used for assessing the quality of non-randomized studies. Risk of bias and quality assessment was conducted by two independent reviewers. If there is any disagreement, a third reviewer made the decision after a group discussion where both parties were taken into consideration.
Outcomes
The following outcomes were analyzed: postoperative mortality, postoperative complications, anastomotic leak, length of hospital stay, and length of ICU stay.
Results
Study selection and characteristics
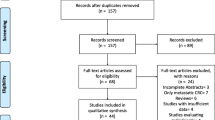
As detailed in the selection flow diagram (Fig. 1), the initial search yielded 2804 results. After the removal of duplicate records and ineligible studies, 103 remained and were fully reviewed based. Of these, 40 were included [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], comprising 5669 patients from retrospective and prospective observational data.
The mean age across the studies was 65 years, with male predominance (82%). The baseline characteristics of the included studies are reported in Table 1.
Quality assessment using the New-castle-Ottawa Scale demonstrated that all the included studies scored 5 or 6 points out of 9 (Supplementary File 1).
Postoperative mortality
Patients with sarcopenia had a similar all-cause mortality rate compared with non-sarcopenic patients after esophagectomy (RD: 0.01; 95% CI: -0.03 to 0.05; I2 = 93.23%; 23 studies with 3573 patients; see Fig. 2).
Postoperative complications
Sarcopenia before the esophageal surgery was related to an increased risk for overall complications (RD: 0.08; 95% CI: 0.02 to 0.14; I2 = 67.69%; 24 studies with 3767 patients; see Fig. 3a), and a higher risk for severe complications (Clavien-Dindo > IIIa) (RD: 0.11; 95% CI: 0.04 to 0.19; I2 = 68.90%; 10 studies with 1489 patients; see Fig. 3b). It was reported an increased risk for pneumonia (RD: 0.13; 95% CI: 0.09 to 0.18; I2 = 63.66%; 21 studies with 3062 patients; see Fig. 4b). However, the anastomotic leakage rate was similar between the two groups (RD 0.01; 95% CI: -0.01 to 0.02; I2 = 0,00%; 28 studies with 4316 patients; see Fig. 4a).
Length of hospital stay
Patients with sarcopenia had a longer length of hospital stay (MD: 3.54 days; 95% CI: 0.41 to 6.66; I2 = 94.82%; 15 studies with 1882 patients; see Fig. 5) than patients with no sarcopenia before esophagectomy.
Overall survival
Patients with sarcopenia had a lower probability of survival at 3-year follow-up (RD: -0.16; 95% CI: -0.23 to -0.10; I2 = 70.35%; 24 studies with 3504 patients, see Fig. 6).
Sensitivity analysis
A subset analysis of studies that assessed sarcopenia using a cutoff for SMI ≤ 38.5 cm2/m2 in women and ≤ 52.4 cm2/m2 in men showed a reduction in I2 values. The direction and significance of the results were consistent for all endpoints except postoperative overall complications. The subgroup analyses found a significant impact of sarcopenia on overall complications, both in the fixed and random effect models. (Supplementary File 2).
Discussion
In this systematic review and meta-analysis, we compared sarcopenic with non-sarcopenic patients who underwent esophagectomy for cancer. Preoperative sarcopenia was related to poor short- and long-term postoperative outcomes.
A variety of methods have been used to evaluate nutrition in esophageal cancer patients. Anthropometric measurements, blood indicators, energy expenditure, validated nutritional risk score, and patient-reported dietary history could be generally categorized among them [55, 56]. However, blood biomarkers of malnutrition may be affected by systemic therapies, and anthropometric measurements may fail in detecting early signs of muscle loss or in detecting malnutrition among patients with fluid disturbance, such as those with hypoalbuminemia [57, 58]. The current review focuses on the assessment of muscle mass.
The decrease in skeletal muscle mass, strength, and physical performance, known as sarcopenia, has been linked to several consequences in the human body [59, 60], making patients vulnerable to adverse outcomes. Muscle tissue is essential for protein storage, regulation of glucose metabolism, the balance of hormones, and the immunological system, aside from mobilization [61].
Our review showed that sarcopenia before oncological esophagectomy was linked to a higher risk for postoperative complications, mainly pneumonia. However, it not only negatively influenced esophageal cancer, but also the other types of cancer surgery [62, 63]. Weakening of the muscles responsible for changing the volume of the thoracic cavity during respiration may favor low thoracic expansibility during the postoperative period, which in turn leads to a higher risk for atelectasis, pleural effusion, and pneumonia [64]. Besides, loss of thoracic wall muscles may also contribute to extubation failure and prolonged mechanical ventilation [65]. The length of mechanical ventilation is directly related to the risk of ventilator-associated pneumonia. Chastre et al. [66] showed that the cumulative risk for pneumonia caused by Acinetobacter spp. in patients under mechanical ventilation is 3.4, 20, and 48% at 10, 20, and 30 days after the intubation, respectively.
Generalized sarcopenia of skeletal muscles also reflects in swallowing muscles. This condition is named sarcopenic dysphagia [67]. Loss of strength in the swallowing muscles may also contribute to aspiration pneumonia [68] and enhance perioperative malnutrition due to dysphagia, leading to a vicious cycle of sarcopenic dysphagia and malnutrition.
The limb and trunk skeletal muscle loss also impacts the patient’s capacity for early ambulation. The mobilization is inherently challenging in the postoperative course of an esophagectomy due to the restrictions imposed by thoracic drains, catheters, pumps, central lines, feeding tubes, and pain. Patients who delay mobilization have an increased incidence of pulmonary conditions, infectious complications, extended hospitalization, and a decreased home discharge rate [69, 70]. In addition, bed rest enhances muscle loss and sarcopenia [71], creating another vicious cycle in which patients lack limb strength and immobilization, postponing patients’ recovery from surgery. For this reason, early ambulation is considered one of the cornerstone components of enhanced recovery after surgery (ERAS) protocols [72], it`s recommend early mobilization to improve lung function and tissue oxygenation and avoid thromboembolic events [73]. Additionally, there is also proven evidence of benefits to the patients that enroll in prehabilitation intervention [74, 75]. Especially the multimodal therapy which has a combination of aerobic and resistance exercises, nutritional supplementation and psychological support [76].
Muscle fibers also influence the immunological response by controlling interleukin-6 and other peptides, regulating the synthesis of tumor necrosis factor-alpha and insulin resistance [77]. The reduction in skeletal muscle may cause immunosenescence, which is characterized by decreased cellular immunological function and increased inflammatory activity [78] in response to tumors, releasing pro-inflammatory cytokines and growth factors. A number of inflammatory indicators are reportedly prognostic factors of cancers, including the C-reactive protein-to-albumin ratio, neutrophil-to-lymphocytes ratio, and others [79, 80]. These inflammatory biomarkers are purportedly linked to the long-term survival of several cancer types, including esophageal neoplasms [81,82,83,84]. This inflammatory change might cause decreased host response to cancer [85] and may explain why sarcopenia impairs survival rates, as demonstrated in the current study’s findings.
Sarcopenia is also an indirect finding of the whole malnutrition status, comprising deficiency in the ingested amount of proteins, calories, minerals, and vitamins, all of which are essential for proper immune system function, cancer cells fighting, infections control, and healing processes [86, 87]. Hypoalbuminemia is one of the serum biomarkers of inadequate protein intake [88], and its relationship to unfavorable surgical results has been well established [89]. Albumin is involved in a range of physiological processes in the human body, including fluid kinetics and metabolism, and consequently, its deficiency is associated with numerous adverse postoperative outcomes [90]. Joliat et al. [91], in a recently published systematic review evaluating outcomes in gastrointestinal surgery, showed that low serum albumin was related to wound-related complications, acute respiratory distress syndrome, acute kidney injury, sepsis, anastomotic leak, ileus, and others.
In this sense, it is essential to discuss the available interventions for sarcopenia prevention, treatment, and decreasing its process before esophagectomy. Every esophageal cancer patient planning to undergo esophagectomy should be thoroughly evaluated for sarcopenia, where sarcopenia examination and severity classification should be purposefully undertaken to contemplate some prehabilitation strategies that aim to reverse the sarcopenia status before the surgery [92].
This study has some limitations. The definition of sarcopenia and the methodologies applied for measuring body composition employed in each study were heterogeneous, which is one of the study’s shortcomings. Several methods for evaluating sarcopenia have been proposed, such as lumbar skeletal muscle index, skeletal muscle mass index, psoas muscle index, low appendicular skeletal muscle mass index, and others. Besides, the cut point for differentiating sarcopenic and non-sarcopenic patients is still not well established. Most of the included studies used different cut points for women and men, considering the likely differences in muscle mass between these groups. The most frequently reported parameter and cutoff value used was lumbar skeletal muscle index (SMI) ≤ 38.5 cm2/m2 in women and ≤ 52.4 cm2/m2 in men. In a subgroup analysis, using only studies that applied this cut point for SMI, the statistical heterogeneity was reduced. However, other demographic variables aside from sex might also impact muscle mass, including ethnicity, age, and comorbidities, all contributing to clinical heterogeneity among the studies. Considering the presumed clinical heterogeneity, we used the random effect as the primary analysis model. However, sensitivity analysis with the fixed effect model in the subgroup analysis was consistent for most endpoints, demonstrating the robustness and validity of our findings, despite the study’s limitations.
Conclusion
Sarcopenia is a highly significant preoperative comorbidity in patients submitted to esophagectomy for cancer. Preoperative sarcopenia imposes a higher risk for overall complications and severe complications. Besides, patients with sarcopenia had a lower chance of long-term survival.
Data Availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- SMM:
-
Skeletal muscle mass
- SMI:
-
Skeletal muscle index
- ICU:
-
Intensive care unit
- RD:
-
Risk difference
- MD:
-
Mean differences
References
Tomaszek S, Cassivi SD. Esophagectomy for the treatment of esophageal cancer. Gastroenterol Clin North Am. 2009;38(1):169–81.
Wang PY, Xu LD, Chen XK, Xu L, Yu YK, Zhang RX, Sun HB, Wu HL, Li Y. Sarcopenia and short-term outcomes after esophagectomy: a meta-analysis. Ann Surg Oncol. 2020;27:3041–51.
Evans RP, Singh P, Nepogodiev D, Bundred J, Kamarajah S, Jefferies B, Siaw-Acheampong K, Wanigasooriya K, McKay S, Mohamed I, Whitehouse T. Study protocol for a multicenter prospective cohort study on esophagogastric anastomoses and anastomotic leak (the Oesophago-Gastric anastomosis Audit/OGAA). Dis Esophagus. 2020;33(1):doz007.
Schmidt HM, Gisbertz SS, Moons J, Rouvelas I, Kauppi J, Brown A, Asti E, Luyer M, Lagarde SM, Berlth F, Philippron A, Bruns C, Hölscher A, Schneider PM, Raptis DA, van Berge Henegouwen MI, Nafteux P, Nilsson M, Räsanen J, Palazzo F, Rosato E, Mercer S, Bonavina L, Nieuwenhuijzen G, Wijnhoven PL, Schröder W, Pattyn P, Grimminger PP, Gutschow CA. Defining benchmarks for Transthoracic Esophagectomy: a Multicenter analysis of total minimally invasive esophagectomy in low risk patients. Ann Surg. 2017;266(5):814–21. https://doi.org/10.1097/SLA.0000000000002445. PMID: 28796646.
Huddy JR, Huddy FM, Markar SR, Tucker O. Nutritional optimization during neoadjuvant therapy prior to surgical resection of esophageal cancer—a narrative review. Dis Esophagus. 2018;31(1):dox110.
Steenhagen E. Preoperative nutritional optimization of esophageal cancer patients. J Thorac disease. 2019;11(Suppl 5):645.
Tustumi F, Kimura CM, Takeda FR, Uema RH, Salum RA, Ribeiro-Junior U, Cecconello I. Prognostic factors and survival analysis in esophageal carcinoma. ABCD Arquivos Brasileiros de Cirurgia Digestiva (São Paulo). 2016;29:138–41.
Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8(3):265–9.
Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. 2018;31(8).
Behne TE, Dock-Nasimento DB, Sierra JC, Rodrigues HH, Palauro ML, Andreo FO, DE-Aguilar-Nascimento JE. Association between preoperative potential sarcopenia and survival of cancer patients undergoing major surgical procedures. Volume 47. Revista do Colégio Brasileiro de Cirurgiões; 2020.
McGovern J, Dolan RD, Horgan PG, Laird BJ, McMillan DC. Computed tomography-defined low skeletal muscle index and density in cancer patients: observations from a systematic review. J Cachexia Sarcopenia Muscle. 2021;12(6):1408–17.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Booth A, Avey M, de Vries R, Moher D, Stewart L. PROSPERO International prospective register of systematic reviews: An expanding resource. In3rd International Symposium on Systematic Review and Meta-Analysis of Laboratory Animal Studies 2014 Nov.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Colcord ME, Benbow JH, Trufan S, Gower NL, Byrne ME, Shea RE, Watson MD, Hill JS, Squires MH, Salo JC. Preoperative muscle strength is a predictor of outcomes after esophagectomy. J Gastrointest Surg. 2021;25:3040–8.
Elliott JA, Doyle SL, Murphy CF, King S, Guinan EM, Beddy P, Ravi N, Reynolds JV. Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg. 2017;266(5):822–30.
Fehrenbach U, Wuensch T, Gabriel P, Segger L, Yamaguchi T, Auer TA, Beetz NL, Denecke C, Kröll D, Raakow J, Knitter S. CT body composition of sarcopenia and sarcopenic obesity: predictors of postoperative complications and survival in patients with locally advanced esophageal adenocarcinoma. Cancers. 2021;13(12):2921.
Grotenhuis BA, Shapiro J, Van Adrichem S, de Vries M, Koek M, Wijnhoven BP, van Lanschot JJ. Sarcopenia/muscle mass is not a prognostic factor for short-and long-term outcome after esophagectomy for cancer. World J Surg. 2016;40:2698–704.
Harada K, Ida S, Baba Y, Ishimoto T, Kosumi K, Tokunaga R, Izumi D, Ohuchi M, Nakamura K, Kiyozumi Y, Imamura Y. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. 2016;29(6):627–33.
Ida S, Watanabe M, Yoshida N, Baba Y, Umezaki N, Harada K, Karashima R, Imamura Y, Iwagami S, Baba H. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann Surg Oncol. 2015;22:4432–7.
Ishida T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, Kurokawa Y, Motoori M, Kimura Y, Nakajima K, Mori M. Impact of measurement of skeletal muscle mass on clinical outcomes in patients with esophageal cancer undergoing esophagectomy after neoadjuvant chemotherapy. Surgery. 2019;166(6):1041–7.
Järvinen T, Ilonen I, Kauppi J, Salo J, Räsänen J. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol. 2018;16:1–9.
Kamada T, Ohdaira H, Ito E, Fuse Y, Takahashi J, Nakashima K, Nakaseko Y, Yoshida M, Eto K, Suzuki Y. Preoperative masseter muscle sarcopenia predicts mortality in patients with oesophageal cancer. Anticancer Res. 2022;42(1):301–10.
Kamitani N, Migita K, Matsumoto S, Wakatsuki K, Kunishige T, Nakade H, Miyao S, Sho M. Association of skeletal muscle loss with the long-term outcomes of esophageal cancer patients treated with neoadjuvant chemotherapy. Surg Today. 2019;49:1022–8.
Kawakita Y, Motoyama S, Sato Y, Wakita A, Nagaki Y, Imai K, Minamiya Y. Decreases in the psoas muscle index correlate more strongly with survival than other prognostic markers in esophageal cancer after neoadjuvant chemoradiotherapy plus esophagectomy. World J Surg. 2020;44:1559–68.
Kudou K, Saeki H, Nakashima Y, Edahiro K, Korehisa S, Taniguchi D, Tsutsumi R, Nishimura S, Nakaji Y, Akiyama S, Tajiri H. Prognostic significance of sarcopenia in patients with esophagogastric junction cancer or upper gastric cancer. Ann Surg Oncol. 2017;24:1804–10.
Kurita D, Utsunomiya D, Kubo K, Fujii Y, Kanematsu K, Ishiyama K, Oguma J, Daiko H. Handgrip strength predicts early postoperative dysphagia after thoracoscopic–laparoscopic esophagectomy in male patients with esophageal cancer. Esophagus. 2022;19(4):586–95.
Maeda N, Shirakawa Y, Tanabe S, Sakurama K, Noma K, Fujiwara T. Skeletal muscle loss in the postoperative acute phase after esophageal cancer surgery as a new prognostic factor. World J Surg Oncol. 2020;18:1–0.
Makiura D, Ono R, Inoue J, Kashiwa M, Oshikiri T, Nakamura T, Kakeji Y, Sakai Y, Miura Y. Preoperative sarcopenia is a predictor of postoperative pulmonary complications in esophageal cancer following esophagectomy: a retrospective cohort study. J geriatric Oncol. 2016;7(6):430–6.
Makiura D, Ono R, Inoue J, Fukuta A, Kashiwa M, Miura Y, Oshikiri T, Nakamura T, Kakeji Y, Sakai Y. Impact of sarcopenia on unplanned readmission and survival after esophagectomy in patients with esophageal cancer. Ann Surg Oncol. 2018;25:456–64.
Matsunaga T, Miyata H, Sugimura K, Motoori M, Asukai K, Yanagimoto Y, Takahashi Y, Tomokuni A, Yamamoto K, Akita H, Nishimura J. Prognostic significance of sarcopenia and systemic inflammatory response in patients with esophageal cancer. Anticancer Res. 2019;39(1):449–58.
Mayanagi S, Ishikawa A, Matsui K, Matsuda S, Irino T, Nakamura R, Fukuda K, Wada N, Kawakubo H, Hijikata N, Ando M. Association of preoperative sarcopenia with postoperative dysphagia in patients with thoracic esophageal cancer. Dis Esophagus. 2021;34(9):doaa121.
Menezes TM, Dias MO, Dos Reis R, Elias J Jr, Lucchesi FR, Araujo RL. Prognostic value of muscle depletion assessed by computed tomography for surgical outcomes of cancer patients undergoing total esophagectomy and gastrectomy. J Surg Oncol. 2020;121(5):814–22.
Murnane LC, Forsyth AK, Koukounaras J, Pilgrim CH, Shaw K, Brown WA, Mourtzakis M, Tierney AC, Burton PR. Low muscularity increases the risk for post-operative pneumonia and delays recovery from complications after oesophago‐gastric cancer resection. ANZ J Surg. 2021;91(12):2683–9.
Nagata K, Tsujimoto H, Nagata H, Harada M, Ito N, Kanematsu K, Nomura S, Horiguchi H, Hiraki S, Hase K, Yamamoto J. Impact of reduced skeletal muscle volume on clinical outcome after esophagectomy for esophageal cancer: a retrospective study. Medicine. 2018;97(30).
Nakashima Y, Saeki H, Nakanishi R, Sugiyama M, Kurashige J, Oki E, Maehara Y. Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. 2018;267(6):1100–4.
Nambara M, Miki Y, Tamura T, Yoshii M, Toyokawa T, Tanaka H, Lee S, Muguruma K, Shibata T, Ohira M. The optimal definition of sarcopenia for predicting postoperative pneumonia after esophagectomy in patients with esophageal cancer. World J Surg. 2021;45(10):3108–18.
Nishigori T, Okabe H, Tanaka E, Tsunoda S, Hisamori S, Sakai Y. Sarcopenia as a predictor of pulmonary complications after esophagectomy for thoracic esophageal cancer. J Surg Oncol. 2016;113(6):678–84.
Oguma J, Ozawa S, Kazuno A, Yamamoto M, Ninomiya Y, Yatabe K. Prognostic significance of sarcopenia in patients undergoing esophagectomy for superficial esophageal squamous cell carcinoma. Dis Esophagus. 2019;32(7):doy104.
Paireder M, Asari R, Kristo I, Rieder E, Tamandl D, Ba-Ssalamah A, Schoppmann SF. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol (EJSO). 2017;43(2):478–84.
Panje CM, Höng L, Hayoz S, Baracos VE, Herrmann E, Garcia Schüler H, Meier UR, Henke G, Schacher S, Hawle H, Gérard MA. Skeletal muscle mass correlates with increased toxicity during neoadjuvant radiochemotherapy in locally advanced esophageal cancer: a SAKK 75/08 substudy. Radiat Oncol. 2019;14(1):1–7.
Saeki H, Nakashima Y, Kudou K, Sasaki S, Jogo T, Hirose K, Edahiro K, Korehisa S, Taniguchi D, Nakanishi R, Kubo N. Neoadjuvant chemoradiotherapy for patients with cT3/nearly T4 esophageal cancer: is sarcopenia correlated with postoperative complications and prognosis? World J Surg. 2018;42:2894–901.
Sakai M, Sohda M, Saito H, Ubukata Y, Nakazawa N, Kuriyama K, Hara K, Sano A, Ogata K, Yokobori T, Shirabe K. Impact of combined assessment of systemic inflammation and presarcopenia on survival for surgically resected esophageal cancer. Am J Surg. 2021;221(1):149–54.
Siegal SR, Dolan JP, Dewey EN, Guimaraes AR, Tieu BH, Schipper PH, Hunter JG. Sarcopenia is not associated with morbidity, mortality, or recurrence after esophagectomy for cancer. Am J Surg. 2018;215(5):813–7.
Soma D, Kawamura YI, Yamashita S, Wake H, Nohara K, Yamada K, Kokudo N. Sarcopenia, the depletion of muscle mass, an independent predictor of respiratory complications after oncological esophagectomy. Dis Esophagus. 2019;32(3):doy092.
Srpcic M, Jordan T, Popuri K, Sok M. Sarcopenia and myosteatosis at presentation adversely affect survival after esophagectomy for esophageal cancer. Radiol Oncol. 2020;54(2):237–46.
Sugimura K, Miyata H, Kanemura T, Takeoka T, Shinnno N, Yamamoto K, Omori T, Motoori M, Ohue M, Yano M. Impact of preoperative skeletal muscle mass and physical performance on short-term and long‐term postoperative outcomes in patients with esophageal cancer after esophagectomy. Annals of Gastroenterological Surgery. 2022;6(5):623–32.
Tamandl D, Paireder M, Asari R, Baltzer PA, Schoppmann SF, Ba-Ssalamah A. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol. 2016;26:1359–67.
Uemura S, Shichinohe T, Kurashima Y, Ebihara Y, Murakami S, Hirano S. Effects of preoperative psoas muscle index and body mass index on postoperative outcomes after video-assisted esophagectomy for esophageal cancer. Asian J endoscopic Surg. 2021;14(4):739–47.
Wakefield CJ, Hamati F, Karush JM, Arndt AT, Geissen N, Liptay MJ, Borgia JA, Basu S, Seder CW. Sarcopenia after induction therapy is associated with reduced survival in patients undergoing esophagectomy for locally-advanced esophageal cancer. J Thorac Disease. 2021;13(2):861.
Wang PY, Chen XK, Liu Q, Yu YK, Xu L, Liu XB, Zhang RX, Wang ZF, Li Y. Highlighting sarcopenia management for promoting surgical outcomes in esophageal cancers: evidence from a prospective cohort study. Int J Surg. 2020;83:206–15.
Watanabe A, Oshikiri T, Sawada R, Harada H, Urakawa N, Goto H, Hasegawa H, Kanaji S, Yamashita K, Matsuda T, Makiura D. Actual sarcopenia reflects poor prognosis in patients with esophageal cancer. Ann Surg Oncol. 2022;29(6):3670–81.
Xu J, Zheng B, Zhang S, Zeng T, Chen H, Zheng W, Chen C. Effects of preoperative sarcopenia on postoperative complications of minimally invasive oesophagectomy for oesophageal squamous cell carcinoma. J Thorac disease. 2019;11(6):2535.
Yassaie SS, Keane C, French SJ, Al-Herz FA, Young MK, Gordon AC. Decreased total psoas muscle area after neoadjuvant therapy is a predictor of increased mortality in patients undergoing oesophageal cancer resection. ANZ J Surg. 2019;89(5):515–9.
van Stijn MF, Korkic-Halilovic I, Bakker MS, van der Ploeg T, van Leeuwen PA, Houdijk AP. Preoperative nutrition status and postoperative outcome in elderly general surgery patients: a systematic review. J Parenter Enter Nutr. 2013;37(1):37–43.
Madden AM, Smith S. Body composition and morphological assessment of nutritional status in adults: a review of anthropometric variables. J Hum Nutr dietetics. 2016;29(1):7–25.
Connolly GC, Khorana AA, Kuderer NM, Culakova E, Francis CW, Lyman GH. Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res. 2010;126(2):113–8.
Purnak T, Yilmaz Y. Liver disease and malnutrition. Best Pract Res Clin Gastroenterol. 2013;27(4):619–29.
Marcell TJ. Sarcopenia: causes, consequences, and preventions. The journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58(10):M911–6.
Soares JD, Howell SL, Teixeira FJ, Pimentel GD. Dietary amino acids and immunonutrition supplementation in cancer-induced skeletal muscle mass depletion: a mini-review. Curr Pharm Design. 2020;26(9):970–8.
Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154(3):557–68.
Traeger L, Bedrikovetski S, Nguyen TM, Kwan YX, Lewis M, Moore JW, Sammour T. The impact of preoperative sarcopenia on postoperative ileus following colorectal cancer surgery. Tech Coloproctol. 2023 May 15. doi: https://doi.org/10.1007/s10151-023-02812-3. Epub ahead of print. PMID: 37184771.
Shimura M, Mizuma M, Motoi F, Kusaka A, Aoki S, Iseki M, Inoue K, Douchi D, Nakayama S, Miura T, Ishida M, Ohtsuka H, Nakagawa K, Morikawa T, Kamei T, Unno M. Negative prognostic impact of sarcopenia before and after neoadjuvant chemotherapy for pancreatic cancer. Pancreatology. 2023;23(1):65–72. Epub 2022 Nov 28. PMID: 36473785.
Kotta PA, Ali JM. Incentive spirometry for prevention of postoperative pulmonary complications after thoracic surgery. Respir Care. 2021;66(2):327–33.
Woo HY, Oh SY, Lee H, Ryu HG. Evaluation of the association between decreased skeletal muscle mass and extubation failure after long-term mechanical ventilation. Clin Nutr. 2020;39(9):2764–70.
Chastre J, Trouillet JL, Vuagnat A, Joly-Guillou ML, Clavier H, Dombret MC, Gibert C. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157(4):1165–72.
Dellis S, Papadopoulou S, Krikonis K, Zigras F. Sarcopenic dysphagia. A narrative review. J Frailty Sarcopenia Falls. 2018;3(1):1.
Wakabayashi H, Sakuma K. Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle. 2014;5:269–77.
Andersen KS, Skoffer B, Oestergaard LG, Van Tulder M, Petersen AK. The effects of respiratory physiotherapy after lung resection: protocol for a systematic review. Int J Surg protocols. 2017;4:1–5.
Nishijima M, Baba H, Murotani K, Tokai R, Watanabe T, Hirano K, Shibuya K, Hojo S, Matsui K, Yoshioka I, Okumura T. Early ambulation after general and digestive surgery: a retrospective single-center study. Langenbeck’s Archives of Surgery. 2020;405(5):613–22.
Brower RG. Consequences of bed rest. Crit Care Med. 2009;37(10):422–8.
Tazreean R, Nelson G, Twomey R. Early mobilization in enhanced recovery after surgery pathways: current evidence and recent advancements. J Comp Eff Res. 2021;11(2):121–9.
Low DE, Allum W, De Manzoni G, Ferri L, Immanuel A, Kuppusamy M, Law S, Lindblad M, Maynard N, Neal J, Pramesh CS. Guidelines for perioperative care in esophagectomy: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2019;43:299–330.
Kakehi S, Wakabayashi H, Inuma H, Inose T, Shioya M, Aoyama Y, Hara T, Uchimura K, Tomita K, Okamoto M, Yoshida M, Yokota S, Suzuki H. Rehabilitation Nutrition and Exercise Therapy for Sarcopenia. World J Mens Health. 2022;40(1):1–10. https://doi.org/10.5534/wjmh.200190. Epub 2021 Mar 4. PMID: 33831974; PMCID: PMC8761238.
Molenaar CJ, van Rooijen SJ, Fokkenrood HJ, Roumen RM, Janssen L, Slooter GD. Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. Cochrane Database Syst Rev. 2022;5(5):CD013259. doi: https://doi.org/10.1002/14651858.CD013259.pub2. Update in: Cochrane Database Syst Rev. 2023;5:CD013259. PMID: 35588252; PMCID: PMC9118366.
Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, Kaneva P, Augustin B, Wongyingsinn M, Gamsa A, Kim DJ, Vassiliou MC, Feldman LS. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27(4):1072–82. https://doi.org/10.1007/s00464-012-2560-5. Epub 2012 Oct 9. PMID: 23052535.
Pedersen BK, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports. 2003;13(1):56–62.
Bruunsgaard H, Pedersen BK. Effects of exercise on the immune system in the elderly population. Immunol Cell Biol. 2000;78(5):523–31.
Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7.
Tustumi F, Takeda FR, Viyuela MS, da Cruz Junior JB, Brandão AA, Sallum RA, Ribeiro Junior U, Cecconello I. The value of cellular components of blood in the setting of trimodal therapy for esophageal cancer. J Surg Oncol. 2020;121(5):784–94.
Miyata H, Yamasaki M, Kurokawa Y, Takiguchi S, Nakajima K, Fujiwara Y, Mori M, Doki Y. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Experimental and therapeutic medicine. 2011;2(5):879–85.
Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Fushiya N, Koike K, Nishino H, Tajiri H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br J Cancer. 2012;107(6):988–93.
Chen S, Yang X, Feng JF. A novel inflammation-based prognostic score for patients with esophageal squamous cell carcinoma: the c-reactive protein/prognostic nutritional index ratio. Oncotarget. 2016;7(38):62123–32.
Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, Fu JH, Tan ZH, Meng YQ, Fu XY, Ma QL. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterology: WJG. 2015;21(18):5591.
Durham WJ, Dillon EL, Sheffield-Moore M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care. 2009;12(1):72.
Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients. 2019;11(8):1933.
Barchitta M, Maugeri A, Favara G, Magnano San Lio R, Evola G, Agodi A, Basile G. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin. Int J Mol Sci. 2019;20(5):1119.
Seiler WO. Clinical pictures of malnutrition in ill elderly subjects. Nutrition. 2001;17(6):496–8.
Meyer CP, Rios-Diaz AJ, Dalela D, Ravi P, Sood A, Hanske J, Chun FK, Kibel AS, Lipsitz SR, Sun M, Trinh QD. The association of hypoalbuminemia with early perioperative outcomes–a comprehensive assessment across 16 major procedures. Am J Surg. 2017;214(5):871–83.
Haskins IN, Baginsky M, Amdur RL, Agarwal S. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. 2017;36(5):1333–8.
Joliat GR, Schoor A, Schäfer M, Demartines N, Hübner M, Labgaa I. Postoperative decrease of albumin (∆Alb) as early predictor of complications after gastrointestinal surgery: a systematic review. Perioperative Med. 2022;11(1):1–8.
Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92(8):715 – 27. doi: https://doi.org/10.1097/PHM.0b013e31829b4afe. PMID: 23756434.
Acknowledgements
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Study concepts: Francisco Tustumi; Study design: Francisco Tustumi; Data acquisition: Amanda Park; Quality control of data and algorithms: Amanda Park and Daniel José Szor; Data analysis and interpretation: Marina Feliciano Orlandini and Ulysses Ribeiro Junior; Statistical analysis: Marina Feliciano Orlandini and Daniel José Szor; Manuscript preparation: Marina Feliciano Orlandini; Manuscript editing: Amanda Park; Manuscript review: Amanda Park and Ulysses Ribeiro Junior. All authors have read and approved the publication of the study.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflict of interest.
Ethics approval and consent to participate
The local institutional review board waived ethical approval and consent to participate for this study due to the review design of the manuscript.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12893_2023_2149_MOESM1_ESM.docx
Additional File 1: Subgroup analysis included only studies using Skeletal Muscle Mass Index (SMI) for assessing sarcopenia
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Park, A., Orlandini, M.F., Szor, D.J. et al. The impact of sarcopenia on esophagectomy for cancer: a systematic review and meta-analysis. BMC Surg 23, 240 (2023). https://doi.org/10.1186/s12893-023-02149-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-023-02149-6