Abstract
Background
This systematic review and meta-analysis aimed to study the evidence on the efficacy and safety of omitting axillary lymph node dissection (ALND) for patients with clinically node-negative but sentinel lymph node (SLN)-positive breast cancer using all the available evidence.
Methods
The Embase, Medline, and Cochrane Library databases were searched through February 25, 2023. Original trials that compared only the sentinel lymph node biopsy (SLNB) with ALND as the control group for patients with clinically node-negative but SLN-positive breast cancer were included. The primary outcomes were axillary recurrence rate, total recurrence rate, disease-free survival (DFS), and overall survival (OS). Meta-analyses were performed to compare the odds ratio (OR) in rates and the hazard ratios (HR) in time-to-event outcomes between both interventions. Based on different study designs, tools in the revised Cochrane risk of bias tool were used for randomized trials and the risk of bias in nonrandomized studies of interventions to assess the risk of bias for each included article. Funnel plots and Egger's test were used for the publication’s bias assessment.
Results
In total, 30 reports from 26 studies were included in the systematic review (9 reports of RCTs, 21 reports of retrospective cohort studies). According to our analysis, omitting ALND in patients with clinically node-negative but SLN-positive breast cancer had a similar axillary recurrence rate (OR = 0.95, 95% confidence interval (CI): 0.76–1.20), DFS (HR = 1.02, 95% CI: 0.89–1.16), and OS (HR = 0.97, 95% CI: 0.92–1.03), but caused a significantly lower incidence of adverse events and benefited in locoregional recurrence rate (OR = 0.76, 95% CI: 0.59–0.97) compared with ALND.
Conclusion
For patients with clinically node-negative but SLN-positive breast cancer (no matter the number of the positive SLN), this review showed that SLNB alone had a similar axillary recurrence rate, DFS, and OS, but caused a significantly lower incidence of adverse events and showed a benefit for the locoregional recurrence compared with ALND. An OS benefit was found in the Macro subset that used SLNB alone versus complete ALND. Therefore, omitting ALND is feasible in this setting.
Trial registration
CRD 42023397963
Similar content being viewed by others
Background
Axillary lymph node dissection (ALND) has been one of the standard treatments for breast cancer during the twentieth century to prevent the dissemination of breast cancer [1]. However, the management of the axilla in breast cancer has evolved recently, with sentinel lymph node biopsy (SLNB) becoming the standard of care for patients clinically negative for axillary lymph node metastasis [2,3,4]. For patients with negative sentinel lymph node (SLN), omitting ALND was the consistent choice for most management guidelines [5,6,7]. In contrast, patients with positive SLN were thought to be at risk of further axillary metastases or decreased overall survival (OS) [8]. The consistent guideline on this was as follows: 1) if positive sentinel lymph node for micrometastasis, no further axillary surgery. Besides, if the patients were 1) no preoperative systemic therapy, 2) tumor size < 5 cm, 3) ≤ 2 positive sentinel lymph nodes, 4) breast-conserving therapy planned, and 5) whole-breast radiation planned [9]. However, ALND has traditionally been advocated for patients with positive SLN [10]. Furthermore, it was reported that ALND may be safely spared in a select cohort of patients [11].
The role of ALND is questioned for disease-free survival (DFS) and OS for selected SLNB positive patients compared with SLNB alone. The Z0011 trial carried out by Giuliano et al., indicated that SLNB without ALND could offer excellent regional control and survival for selected patients with early-stage breast cancer [12,13,14]. It might be feasible to omit ALND in selected patients with positive SLNB. However, there is no evidence on the optimal management of these patients for patient selection according to the histopathological classification.
The histopathological classification of SLN metastasis included macrometastasis (Macro), micrometastasis (Micro), and isolated tumor cells (ITCs) [15, 16]. Originally, nodal metastasis of > 2 mm in largest diameter was defined as Macro, metastasis between > 0.2 mm but ≤ 2.0 mm was defined as Micro and metastasis of ≤ 0.2 mm was defined as ITCs. The prognostic results refer to the different histopathological classification (Macro versus Micro/ITCs) should be different based on their biological characteristics [17].
Some systematic reviews focused on the efficacy of omitting ALND for selected patients with positive SLN [10, 18,19,20]. However, few have reported the safety of the procedure. In addition, most focused on studies reported 5 years ago and some meaningful observational studies were excluded. However, no study has tried to explore the difference between different histopathological categories of SLN metastasis. The present systematic review and meta-analysis aimed to study the evidence in the literature on the efficacy and safety of omitting ALND for patients with clinically negative axillary lymph node metastasis but positive SLN using all the available evidence. In addition, the feasibility of patient selection for the histopathological classification of SLN metastasis was studied to provide further useful evidence for decision-making in practice.
Methods
The PRISMA statement was followed for systematic reviews and meta-analyses to report the present study [21, 22]. An ethics review was waived due to the retrospective and anonymous characteristics of the study.
Criteria for considering studies for this review
The study inclusion and exclusion criteria included: (1) original comparative research studies in full reports, including retrospective or prospective studies and randomized controlled trials (RCTs). Letters, commentaries, conference abstracts, or reviews were excluded; (2) patients with clinically node-negative breast cancer; (3) patients with SLNB positive early-stage breast cancer. Papers with no information of SLNB were excluded; (4) SLNB without ALND as the experimental group; (5) ALND as the control group; and (6) data on any of the following outcomes: OS, progressive-free survival, local recurrence, and adverse events (e.g., lymphedema, sensory neuropathy, motor neuropathy, and infection). In addition, papers without information based on the outcomes or the data that could not be analyzed were excluded.
Search strategies and study selection
The electronic databases searched to identify reports of relevant clinical trials included the Cochrane CENTRAL Register of Controlled Trials in the Cochrane Library (searched on 25 February 2023), Ovid MEDLINE (1946 to 05 August 2021), and Ovid Embase (1974 to 25 February 2023). The key search terms included sentinel lymph node biopsy (SLNB), breast neoplasms, and lymph node excision. The details of the search strategies can be found in the Additional file 1. In addition, potentially eligible studies were identified by searching the reference lists of retrieved papers.
After omitting duplicated studies, two independent reviewers performed the title and abstract screening for potentially eligible studies using Endnote version X9. Then, full texts for all potentially eligible studies were retrieved to identify studies that met the inclusion and exclusion criteria. Disagreements between both reviewers were referred to a third party.
Outcomes
The primary outcomes included axillary recurrence rate, the total recurrence (e.g., axillary recurrence, local recurrence, and distance metastasis) rate, DFS, and OS. The second outcome was adverse events.
Data collection
Data collection was carried out independently using the pre-test data collection form. Data collection referred to the information of the included studies, which included study design, publication year, country, study design, target population, age of related patients, clinical tumor stages, hormone receptors, nodal metastasis (micro or macro), experimental and control intervention, other interventions, and outcomes.
Study quality assessment
The RCTs’ quality was assessed using the revised Cochrane risk of bias tool for randomized trials (RoB2) and the quality of nonrandomized studies used the risk of bias in nonrandomized studies of interventions (ROBIN-I) tool [23,24,25].
Data analysis
The continuous variables were described with mean and standard deviation (SD), and categorical variables with count with percentage/proportion. For dichotomous data, summary estimates were expressed as odds ratio (OR) with 95% CI (this was carried out for the axillary recurrence rate, total recurrence rate, total survival rate, and adverse events rate). For time-to-event data, the summary estimates were presented as hazard ratio (HR) with 95% CI (this was carried out for time to DFS and time to OS).
For missing data, the study authors were contacted. Then, if the data was insufficient for analysis, descriptive data were presented in the systematic review. Missing data were not used; therefore, studies were excluded that did not have any available data for outcome analysis.
Data analysis was performed using Stata, version 15.0 (Stata Corp. Texas, USA), and Review Manager (Revman) 5.4.1 software. Meta-analysis was performed for outcome measures if ≥ 2 clinically homogenous studies (e.g., studies with similar participants, interventions, and outcomes). Heterogeneity was estimated using the Q-test and I2 score. When the p-value was < 0.1 (for Q-test) and I2 > 50%, the result was considered with heterogeneity, and the random-effects model was used for analysis. Otherwise, a fixed-effects model was applied for analysis [26,27,28]. A p-value < 0.05 was set as the threshold for statistical significance. Subgroup analysis was performed according to the classification of the nodal metastasis (Micro and ITCs versus Macro). Sensitivity analysis was performed according to the methodology quality of the included papers.
Funnel plots generated by Revman 5 confirmed symmetry for the publication bias. If there was an asymmetry of the funnel plot, Egger’s test using Stata version 15 was performed. If the p-value < 0.05 of the Egger’s test, which suggested the publication bias existed, this was dealt with using the trim-and-fill method [29].
Results
Study selection and characteristics of included studies
The flow chart of eligible study selection is shown in Fig. 1. A total of 13,273 studies (1306 studies from Cochrane Library CENTRAL, 5,006 from Medline, 6,961 from Embase, and 5 studies from other sources) were found. After excluding duplications, 10,339 studies were used for the title and abstract screening. Then, 72 papers were retrieved for full-text review. Finally, 30 reports for 26 studies were included in the systematic research and meta-analysis according to the inclusion and exclusion criteria [12, 13, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57].
PRISMA flow chart of the procedure for eligible study selection [50]
In total, 191,329 patients were involved in the included 26 studies with a sample size from 81 to 26,986 patients. The number of the RCTs and retrospective studies was 5 and 21, respectively. The papers were published between 2007 and 2022. However, not all studies supplied the information of the number of the positive SLN and the previous systematic therapy. The details of the included 26 studies reported in 30 papers are listed in Table 1.
Methodological quality appraisal
The risk of bias in three RCTs is summarized in Table 2. Three of the studies had issues with bias in randomization. Therefore, for the overall assessment, two studies (EORTC 10981–22,023 AMAROS Trial and ACOSOG Z0011) were defined as low risk of bias, and the other three (SINODAR-ONE trial, IBCSG 23–01, and AATRM 048/13/2000) were defined as having some concerns.
For the 21 retrospective studies, the risk of bias assessment results is listed in Table 3. All 21 studies have a moderate risk of bias in the selection of the reported result as none of those retrospective studies had priori protocol published. However, no evidence showed that they had problems in the domains with bias in the selection of participants into the study, bias in the classification of interventions, or bias in the measurement of outcomes. In addition, about half of the studies were defined as serious risk of bias due to the confounding (11/21, 52.4%) and with no information on missing data (11/21, 52.4%). Therefore, the overall risk of bias was defined as moderate and serious risk of bias in 10 (47.6%) and 11 (52.4%) studies, respectively.
Axillary recurrence rate
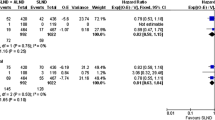
Thirteen papers reported data on the comparison of the axillary recurrence rate between both interventions [13, 31,32,33, 38,39,40,41, 46, 48, 49, 53, 56]. The pooling results showed no statistical difference in axillary recurrence rate between both interventions (p = 0.69). Compared with the ALND group, the OR of axillary recurrence rate for the SLNB group was 0.95 (95% CI: 0.75–1.20) with a fixed model as p = 0.64 (Q-test) and I2 = 0%. The subgroup analysis based on the nodal classification showed that although the mean of OR for the Micro/ITC group (1.21, 95% CI: 0.70–2.09) was higher than the Macro group (0.91, 95% CI: 0.68–1.21), there was no statistically significant difference between them (Fig. 2A).
For sensitivity analysis, after deleting the subsets (e.g., the Micro/ITC group and the Macro group), comparing the SLNB group with the ALND group, the OR of the axillary recurrence rate was 0.91 (95% CI: 0.53–1.56). Compared with the original meta-analysis result, there was no statistically significant difference (Fig. 2A). In addition, based on the funnel plot analysis (Fig. 2B), there was no publication bias in the included studies for this outcome.
Locoregional recurrence rate
Thirteen papers compared the locoregional recurrence rate between both interventions [12, 13, 35, 36, 38, 40, 41, 46, 48, 49, 52, 53, 56]. The pooling results showed benefit for the SLNB group in locoregional recurrence rate (p = 0.03). Compared with the ALND group, the OR of locoregional recurrence rate for the SLNB group was 0.76 (95% CI: 0.59–0.97) with the random model as p = 0.03 (Q-test) and I2 = 45% (Fig. 3A). For patients with the Micro/ITC metastasis, the OR of the locoregional recurrence rate for the SLNB group compared with the ALND group was 1.02 (95% CI: 0.73–1.43). Only one study reported the locoregional recurrence rate for patients with the Macro metastasis, which showed a benefit for the SLNB group (p = 0.02) [56].
For sensitivity analysis, after deleting the subset of the Micro/ITC group and Macro group, comparing the SLNB group with the ALND group, the OR of locoregional recurrence rate was 0.70 (95% CI: 0.52–0.95), which was statistically significant (Fig. 3A). In addition, based on the funnel plot analysis (Fig. 3B), there might be publication bias in the included studies for this outcome. However, Egger's test showed that no small-study effect existed for the outcome based on the data (p = 0.735). Using the trim-and-fill method, the random effect model showed the result (OR = 0.75, 95% CI: 0.56–0.96, p = 0.04) was similar to the original result.
DFS
Ten papers compared the DFS between patients treated with ALND and SLNB alone[12, 13, 35, 40, 44, 46,47,48, 53, 55]. A fixed-effect statistical model (Q-test: p = 0.75 and I2 = 0%) revealed that the DFS was not significantly different between the SLNB group and ALND group (HR = 1.02, 95% CI: 0.89–1.16, p = 0.79). Three papers compared the DFS between patients in Micro/ITC treated ALND and SLNB only [13, 40, 55]. No statistical difference for the DFS was found between the two interventions (HR = 0.98, 95% CI: 0.75–1.28, p = 0.88) (Fig. 4A).
For sensitivity analysis, after deleting the subset of the Micro/ITC group, comparing the SLNB group with the ALND group, the HR of DFS was 1.03 (95% CI: 0.89–1.19, p = 0.70). The funnel plot showed that there was no publication bias that existed (Fig. 4B).
OS
Fourteen papers compared the OS between patients treated with ALND and SLNB only [13, 31, 34, 37, 39, 40, 42, 44, 48, 50, 51, 53, 55, 57]. A fixed-effect statistical model (Q-test: p = 0.26 and I2 = 16%) revealed that the OS was not significantly different between the SLNB only and ALND groups (HR = 0.97, 95% CI: 0.92–1.03, p = 0.37). Six papers compared the DFS between patients in the Micro/ITC treated ALND and SLNB only groups [13, 31, 39, 40, 55, 57]. No statistical difference for the DFS was found between the two interventions (HR = 1.04, 95% CI: 0.91–1.19, p = 0.57) (Fig. 5A). One paper reported a significant increase in the OS that compared SLNB only with the ALND group in patients with Macro (HR = 0.91, 95% CI: 0.83–1.00, p = 0.04) [31].
For sensitivity analysis, after deleting the subset of the Micro/ITC and Macro groups, comparing the SLNB group with the ALND group, the HR of OS was 1.01 (95% CI: 0.92- 1.10, p = 0.85). In addition, according to the funnel plot analysis (Fig. 5B), no publication bias was found in the included studies for this outcome.
Adverse events
Lymphedema was reported in three papers [13, 30, 53]. The OR of lymphedema rate that compared the SLNB group with the ALND group was 0.35 (95% CI: 0.25–0.49) with p < 0.001. The pooled results from two papers for sensory neuropathy showed that the OR of sensory neuropathy rate that compared the SLNB group with the ALND group was 0.55 (95% CI: 0.39–0.79) with p < 0.001 [13, 41]. Lucci et al. reported the adjusted OR of wound infections, axillary seromas, and axillary paresthesia that compared the SLNB group with the ALND group was 0.33 (95% CI: 0.16–0.68), 0.37 (95% CI: 0.22–0.63), and 0.15 (95% CI: 0.10–0.22), respectively [30]. Based on the data reported by Galimberti et al., the OR of motor neuropathy that compared the SLNB group with the ALND group was 0.33 (95% CI: 0.17–0.62) [13].
Discussion
Based on the meta-analysis of the present review, the risk of axillary recurrence, disease progress, and overall mortality did not increase when patients with clinically node-negative but SLN-positive breast cancer were treated with SLNB only versus SLNB plus ALND. However, the SLNB group shew a benefit in locoregional recurrence than the ALND group does. In addition, the Macro subset of patients showed increased overall mortality (HR = 0.91, 95% CI: 0.83–1.00, p = 0.04). Compared with SLNB only, ALND was associated with an increased risk of adverse events (e.g., lymphoedema, sensory neuropathy, wound infections, axillary seromas, axillary paresthesia, and motor neuropathy) with a statistical significance.
In a meta-analysis published in 2015, Joyce et al. confirmed a significant benefit of ALND in the local control of axillary disease (OR = 2.25, 95% CI: 1.28–3.94, p = 0.0047) and OS (OR = 1.22, 95% CI: 1.03–1.44, p = 0.02) for invasive breast cancer patients [10]. However, the patients included in this meta-analysis were patients undergoing surgery for invasive breast cancer, which did not restrict patients to early-stage disease. According to our analysis, omitting ALND in patients with clinically node-negative but SLN-positive breast cancer had a similar axillary recurrence rate, DFS, and OS, but caused significantly lower incidence of adverse events compared with ALND and showed a benefit for locoregional recurrence. Our findings agreed with Chen et al. [18], although the participants and intervention criteria differed slightly. Therefore, for patients with clinically node-negative breast cancer, with SLN-positive results, omitting ALND is feasible.
Of note, during the subgroup analysis according to the histopathological classification of SLN metastasis, although there was no statistical significance, the mean OR of axillary recurrence (1.21 versus 0.91) between the two interventions was higher in the Micro/ITC subset than the overall group. Similar as the locoregional recurrence (1.02 versus 0.48). Therefore, for the Micro/ITC subset, there was a trend of axillary recurrence and locoregional recurrence with SLNB alone compared with ALND. The cause of this remains unknown, and future large-scale studies to further explore this are required.
Of note, one paper reported a significant increase in OS comparing the SLNB only group with the ALND group in patients in the Macro subset (HR = 0.91, 95% CI: 0.83–1.00, p = 0.04) [31]. This result indicated that for patients in the Macro subset, SLNB only had benefits for OS. However, this requires confirmation.
The advantages of this systematic review include the rigorous methodology followed in study screening, data analysis, and quality assessment. However, this study has some limitations. First, the recurrence and survival of breast cancer were largely dependent on the systematic treatments that the patients received. This was not included in our analysis and will be the focus of future research. Second, there was insufficient data to perform subgroup analysis according to the histopathological classification of SLN metastasis. In particular, the lack of evidence in the Macro subset. Next, not all the studies supply the information of the number of the positive SLN and the neoadjuvant therapy. Though our review selected studies that compared SNLB + ALND to SNLB, we were unable to control other circumstances surrounding the patient’s treatment, such as other surgical techniques and neoadjuvant therapy. Finally, only three studies were RCTs, and most of the included studies were retrospective cohort studies. Therefore, the quality of the evidence could reduce the impact of the findings from this review.
This review confirmed the evidence base for the feasibility of omitting ALND for patients with clinically node-negative but SLN-positive breast cancer. Evidence shows that although the risk of axillary recurrence, disease progress, and overall mortality was not increased when those patients were treated with SLNB alone versus SLNB and ALND, there were benefits of less adverse events and low locoregional recurrence. An OS benefit was found in the Macro subset using SLNB alone versus SLNB and ALND. Future research should focus on exploring the independent predictors for the interventions.
Conclusion
For patients with clinically node-negative but SLN-positive breast cancer (no matter the number of the positive SLN), this review showed that SLNB alone had a similar axillary recurrence rate, DFS, and OS, but caused a significantly lower incidence of adverse events and showed a benefit for the locoregional recurrence compared with ALND. An OS benefit was found in the Macro subset that used SLNB alone versus complete ALND. Therefore, omitting ALND is feasible in this setting.
Availability of data and materials
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- ALND:
-
Axillary lymph node dissection
- SLN:
-
Sentinel lymph node
- SLNB:
-
Sentinel lymph node biopsy
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- OR:
-
Odds ratio
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- Macro:
-
Macrometastasis
- Micro:
-
Micrometastasis
- ITCs:
-
Isolated tumor cells
- RoB2:
-
Risk of bias tool for randomized trials
- ROBIN-I:
-
Risk of bias in nonrandomized studies of interventions
- RCT:
-
Randomized controlled trial
References
Magnoni F, Galimberti V, Corso G, Intra M, Sacchini V, Veronesi P. Axillary surgery in breast cancer: an updated historical perspective. Semin Oncol. 2020;47(6):341–52.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, Weaver DL, Miller BJ, Jalovec LM, Frazier TG, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–8.
Manca G, Rubello D, Tardelli E, Giammarile F, Mazzarri S, Boni G, Chondrogiannis S, Marzola MC, Chiacchio S, Ghilli M, et al. Sentinel lymph node biopsy in breast cancer: indications, contraindications, and controversies. Clin Nucl Med. 2016;41(2):126–33.
Motomura K, Inaji H, Komoike Y, Kasugai T, Nagumo S, Noguchi S, Koyama H. Sentinel node biopsy in breast cancer patients with clinically negative lymph-nodes. Breast Cancer. 1999;6(3):259–62.
Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, et al. Breast cancer, version 3.2020, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452–78.
Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, Hantel A, Henry NL, Muss HB, Smith TJ, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961–5.
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(6):691–722.
Carlo JT, Grant MD, Knox SM, Jones RC, Hamilton CS, Livingston SA, Kuhn JA. Survival analysis following sentinel lymph node biopsy: a validation trial demonstrating its accuracy in staging early breast cancer. Proc (Bayl Univ Med Cent). 2005;18(2):103–7.
Veronesi P, Corso G. Standard and controversies in sentinel node in breast cancer patients. Breast. 2019;48(Suppl 1):S53-s56.
Joyce DP, Manning A, Carter M, Hill ADK, Kell MR, Barry M. Meta-analysis to determine the clinical impact of axillary lymph node dissection in the treatment of invasive breast cancer. Breast Cancer Res Treat. 2015;153(2):235–40.
Tomasicchio G, Mastropasqua MG, Picciariello A, Montanaro AE, Signorile D, Cirilli A, Punzo C. A new possible cut-off of cytokeratin 19 mRNA copy number by OSNA in the sentinel node of breast cancer patients to avoid unnecessary axillary dissection: a 10-year experience in a tertiary breast unit. Cancers (Basel). 2022;14(14):3384.
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75.
Galimberti V, Zurrida S, Luini A, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14(4):297–305.
Huang TW, Su CM, Tam KW. Axillary management in women with early breast cancer and limited sentinel node metastasis: a systematic review and metaanalysis of real-world evidence in the post-acosog z0011 era. Ann Surg Oncol. 2021;28(2):920–9.
Sawaki M, Shien T, Iwata H. TNM classification of malignant tumors (Breast Cancer Study Group). Jpn J Clin Oncol. 2019;49(3):228–31.
Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20(17):3628–36.
Tsuda H. Histological examination of sentinel lymph nodes: significance of macrometastasis, micrometastasis, and isolated tumor cells. Breast Cancer. 2015;22(3):221–9.
Chen WJ, Xie XH, Xu XH, Lv XA, Gao XF, Wang B. Efficacy and safety of omitting axillary lymph node dissection in early breast cancer patients with sentinel-node metastases: a systematic review and meta-analysis. Int J Clin Exp Med. 2018;11(11):11424–33.
Ram R, Singh J, McCaig E. Sentinel node biopsy alone versus completion axillary node dissection in node positive breast cancer: Systematic review and meta-analysis. Int J Breast Cancer. 2014;2014:513780.
Schmidt-Hansen M, Bromham N, Hasler E, Reed MW. Axillary surgery in women with sentinel node-positive operable breast cancer: a systematic review with meta-analyses. Springerplus. 2016;5:85.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339:b2700.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2019;366:l4898.
Hinneburg I. ROBINS-1: a tool for asssessing risk of bias in non-randomised studies of interventions. Med Monatsschr Pharm. 2017;40(4):175–7.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical research ed). 2016;355:i4919.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60.
Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206.
Shi LY, Lin LF. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore). 2019;98(23):e15987–e15987.
Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Giuliano AE. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–63.
Bilimoria KY, Bentrem DJ, Hansen NM, Bethke KP, Rademaker AW, Ko CY, Winchester DP, Winchester DJ. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–53.
Degnim AC, Zakaria S, Boughey JC, Sookhan N, Reynolds C, Donohue JH, Farley DR, Grant CS, Hoskin T. Axillary recurrence in breast cancer patients with isolated tumor cells in the sentinel lymph node [AJCC N0(i+)]. Ann Surg Oncol. 2010;17(10):2685–9.
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–32; discussion 432‐423.
Yi M, Meric-Bernstam F, Mittendorf EA, Kuerer HM, Hwang RF, Bedrosian I, Rourke L, Hunt KK, Giordano SH. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: Experience from the SEER database. Ann Surg Oncol. 2010;17(SUPPL. 3):S343–51.
Gillanders WE, Eberlein TJ, Margenthaler JA, Gao F, Aft RL, Cyr A. Disease recurrence in sentinel node-positive breast cancer patients forgoing axillary lymph node dissection. Ann Surg Oncol. 2012;19(10):3185–91.
Sola M, Fraile M, Alberro JA, Santesteban P, Ramos M, Fabregas R, Moral A, Ballester B, Vidal S. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol. 2013;20(1):120–7.
Park HS, Chae BJ, Song BJ, Jung SS, Han W, Nam SJ, Youn HJ, Ko BK, Kim DW. Effect of axillary lymph node dissection after sentinel lymph node biopsy on overall survival in patients with T1 or T2 node-positive breast cancer: report from the Korean Breast Cancer Society. Ann Surg Oncol. 2014;21(4):1231–6.
Snow R, Reyna C, Johns C, Lee MC, Sun WH, Fulp WJ, Kiluk JV, Laronga C. Outcomes with and without axillary node dissection for node-positive lumpectomy and mastectomy patients. Am J Surg. 2015;210(4):685–93.
Tvedskov TF, Kroman N, Jensen M, Ejlertsen B, Christiansen P, Balslev E. Prognostic significance of axillary dissection in breast cancer patients with micrometastases or isolated tumor cells in sentinel nodes: a nationwide study. Breast Cancer Res Treat. 2015;153(3):599–606.
Houvenaeghel G, Boher JM, Reyal F, Cohen M, Garbay JR, Classe JM, Rouzier R, Giard S, Faure C, Charitansky H, et al. Impact of completion axillary lymph node dissection in patients with breast cancer and isolated tumour cells or micrometastases in sentinel nodes. Eur J Cancer (Oxford, England : 1990). 2016;67:106–18.
Youssef MMG, Cameron D, Pucher PH, Olsen S, Ferguson D. The significance of sentinel lymph node micrometastasis in breast cancer: Comparing outcomes with and without axillary clearance. Breast (Edinburgh, Scotland). 2016;30:101–4.
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The acosog z0011 (alliance) randomized clinical trial. JAMA. 2017;318(10):918–26.
Galimberti V, Vicini E, Colleoni M, Mazzarol G, Cole BF, Viale G, Veronesi P, Massarut S, Zgajnar J, Taffurelli M, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23–01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(10):1385–93.
Lee J, Kim BK, Sun WY, Choi JE, Kim SJ, Lee SB, Seong M-K, Jeong J, Yoon CS. Comparative study between sentinel lymph node biopsy and axillary dissection in patients with one or two lymph node metastases. J Breast Cancer. 2018;21(3):306–14.
Liu YY, Yu TJ, Liu GY. Prognostic significance of further axillary dissection in breast cancer patients with micrometastases & the number of micrometastases: a SEER population-based analysis. Future Sci OA. 2018;4(5):FSO303.
Arisio R, Borella F, Porpiglia M, Durando A, Bellino R, Bau MG, De Sanctis C, Danese S, Benedetto C, Katsaros D. Axillary dissection vs. No axillary dissection in breast cancer patients with positive sentinel lymph node: a single institution experience. In Vivo (Athens, Greece). 2019;33(6):1941–7.
Jung J, Han W, Noh DY, Kim Y, Lee ES, Jung SY, Han JH, Choi HJ, Lee JE, Nam SJ, et al. Retrospectively validating the results of the ACOSOG Z0011 trial in a large Asian Z0011-eligible cohort. Breast Cancer Res Treat. 2019;175(1):203–15.
Kim SS, Ahn SD, Jung JH, Choi EK, Son BH, Ahn SH, Lee JW, Kim HJ, Ko BS, Joo JH. Axillary lymph node dissection does not improve post-mastectomy overall or disease-free survival among breast cancer patients with 1–3 positive nodes. Cancer Res Treat. 2019;51(3):1011–21.
Jung J, Kim BH, Kim J, Lim CS, Hwang KT, Oh S, Kim SJ, Choi IS. Validating the ACOSOG Z0011 trial result: a population-based study using the SEER database. Cancers. 2020;12(4):950.
Kim BK, Park BW, Hur MH, Lee HB, Park MH, Jeong J, Lee HJ, Lee J, Kim D, Sun WY, et al. Omission of axillary lymph node dissection in patients who underwent total mastectomy with 1 or 2 metastatic lymph nodes. Ann Surg Treat Res. 2020;98(6):283–90.
Sun J, Mathias BJ, Laronga C, Sun W, Zhou J-M, Fulp WJ, Kiluk JV, Lee MC. Impact of axillary dissection among patients with sentinel node-positive breast cancer undergoing mastectomy. J Natl Compr Cancer Netw. 2021;19(1):40–7.
Sanvido VM, Elias S, Facina G, Bromberg SE, Nazario ACP. Survival and recurrence with or without axillary dissection in patients with invasive breast cancer and sentinel node metastasis. Sci Rep. 2021;11(1):19893.
Bartels SAL, Donker M, Poncet C, Sauve N, Straver ME, van de Velde CJH, Mansel RE, Blanken C, Orzalesi L, Klinkenbijl JHG, et al. Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981–22023 AMAROS Trial. J Clin Oncol. 2023;41(12):2159–65.
Gao W, Lu S, Zeng Y, Chen X, Shen K. Axilla lymph node dissection can be safely omitted in patients with 1–2 positive sentinel nodes receiving mastectomy: a large multi-institutional study and a systemic meta-analysis. Breast Cancer Res Treat. 2022;196(1):129–41.
Houvenaeghel G, de Nonneville A, Chopin N, Classe JM, Mazouni C, Chauvet MP, Reyal F, Tunon de Lara C, Jouve E, Rouzier R et al. The need to tailor the omission of axillary lymph node dissection to patients with good prognosis and sentinel node micro-metastases. Cancer Med. 2023;12(4):4023–32.
Tinterri C, Gentile D, Gatzemeier W, Sagona A, Barbieri E, Testori A, Errico V, Bottini A, Marrazzo E, Dani C, et al. Preservation of axillary lymph nodes compared with complete dissection in T1–2 breast cancer patients presenting one or two metastatic sentinel lymph nodes: the SINODAR-ONE Multicenter Randomized Clinical Trial. Ann Surg Oncol. 2022;29(9):5732–44.
Zhou Y, Pu S, Jiang S, Li D, Li S, Liu Y, Ren Y, Hao N. The prognostic significance of further axillary dissection for sentinel lymph node micrometastases in female breast cancer: a competing risk analysis using the SEER database. Front Oncol. 2022;12:1012646.
Acknowledgements
Not applicable.
Funding
This study was supported by Natural Science of Liaoning Province of China (No. 20180551104).
Author information
Authors and Affiliations
Contributions
F.Y., Z.H, B.T, Z.X. and Z.Y. conceived the idea of the study; Z.D., W.G. and L.J. analysed the data; F.Y., Z.H, B.T, Z.X. and Z.Y. interpreted the results; F.Y. wrote the paper; all authors discussed the results and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fan, YJ., Li, JC., Zhu, DM. et al. Efficacy and safety comparison between axillary lymph node dissection with no axillary surgery in patients with sentinel node-positive breast cancer: a systematic review and meta-analysis. BMC Surg 23, 209 (2023). https://doi.org/10.1186/s12893-023-02101-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-023-02101-8