Abstract
Background
The relationship between intraoperative lactate levels and prognosis after emergency gastrointestinal surgery remains unclear. The purpose of this study was to investigate the prognostic value of intraoperative lactate levels for predicting in-hospital mortality, and to examine intraoperative hemodynamic managements.
Methods
We conducted a retrospective observational study of emergency GI surgeries performed at our institution between 2011 and 2020. The study group comprised patients admitted to intensive care units postoperatively, and whose intraoperative and postoperative lactate levels were available. Intraoperative peak lactate levels (intra-LACs) were selected for analysis, and in-hospital mortality was set as the primary outcome. The prognostic value of intra-LAC was assessed using logistic regression and receiver operating characteristic (ROC) curve analysis.
Results
Of the 551 patients included in the study, 120 died postoperatively. Intra-LAC in the group who survived and the group that died was 1.80 [interquartile range [IQR], 1.19–3.01] mmol/L and 4.22 [IQR, 2.15–7.13] mmol/L (P < 0.001), respectively. Patients who died had larger volumes of red blood cell (RBC) transfusions and fluid administration, and were administered higher doses of vasoactive drugs. Logistic regression analysis showed that intra-LAC was an independent predictor of postoperative mortality (odds ratio [OR] 1.210, 95% CI 1.070 –1.360, P = 0.002). The volume of RBCs, fluids transfused, and the amount of vasoactive agents administered were not independent predictors. The area under the curve (AUC) of the ROC curve for intra-LAC for in-hospital mortality was 0.762 (95% confidence interval [CI], 0.711–0.812), with a cutoff value of 3.68 mmol/L by Youden index.
Conclusions
Intraoperative lactate levels, but not hemodynamic management, were independently associated with increased in-hospital mortality after emergency GI surgery.
Similar content being viewed by others
Background
Serum lactate levels can be used as a marker for the imbalance between oxygen supply and demand resulting from circulatory impairment [1. ]. In critically ill patients, hyperlactatemia often results from tissue hypoxia due to anaerobic glycolysis [2. ]. Thus, hyperlactatemia can also be a predictor of mortality in critically ill patients [3. , 4. ] as well as after surgery [5. ]. For example, Jung et al. reported that for patients admitted for emergency abdominal surgery, the serum lactate level at the time of admission was predictive of the risk of intra-abdominal infection after surgery [6. ], whereas Crough-Brown et al. demonstrated that the peak serum lactate level within 24 h after emergency gastrointestinal (GI) surgery was predictive of in-hospital mortality [7. ]. Similarly, postoperative serum lactate levels have been shown to be a useful predictor of early outcomes and mortality after surgical treatment of colorectal perforations [8. ]. However, the relationship between intraoperative lactate levels and prognosis after emergency GI surgery remains unclear. Intraoperative lactate levels are expected to vary depending on several factors such as preoperative patient status, the type of surgical procedure, and the degree of hemodynamic management needed for hemorrhage. This study aimed to investigate the hypothesis that, among the various intraoperative factors, intraoperative lactate levels would be a significant predictor of in-hospital death after emergency GI surgery.
Methods
This single-center, retrospective, observational study was conducted at the Nippon Medical School Hospital between 2011 and 2020. This study was approved by the ethics committee of the Nippon Medical School (no. 26–02-427). Informed consent was obtained from our institution’s website as an opt-out option. Data were collected from the medical records.
Patients who had undergone emergency GI surgery except for trauma were enrolled in this study. We only included patients who required admission to intensive care settings and whose intra-and postoperative serum lactate levels were assessed. The criteria for ICU admission were based on the clinician's judgement. First emergency operations were included and cases of second-look procedures, such as an open abdomen strategy after surgery, were excluded to avoid duplication. Cases of laparoscopic surgery were excluded because laparotomy is a well-established approach in our institution, whereas laparoscopic surgery remains a controversial procedure in critically ill patients [9. -11. ]. Blood samples were obtained from arterial catheters at the physician’s discretion. Intraoperative initial and peak lactate levels (initial LACs and intra-LACs, respectively) were selected for analysis (Fig. 1). Postoperative lactate levels (post-LACs) were measured on admission to the intensive care unit after surgery. Intraoperative lactate clearance (LAC-C) was calculated as follows: LAC-C (%) = 100 × {(post-LAC-initial-LAC) / (initial-LAC)}. The primary outcome was overall in-hospital mortality after surgery.
Study design of perioperative lactate measurements. The initial lactate levels (initial LACs) and intraoperative peak lactate levels (intra-LACs) were measured in the operating room. Postoperative lactate levels (post-LACs) were measured upon admission to the intensive care unit. Intraoperative lactate clearance rate (LAC-C) was calculated as follows: LAC-C (%) = 100 × {(post-LA − initial-LAC) / (initial-LAC)}
Transfusion was performed according to the Japanese guidelines [12. ]. Briefly, red blood cell (RBC) transfusion was performed with a hemoglobin (Hb) target of 7–8 g/dL for patients with no heart complications, and approximately 10 g/dL for patients with cardiovascular complications, respiratory disease, or cerebrovascular disorders. The intraoperative maximum vasoactive inotrope score (VIS) was calculated as follows: VIS = dopamine (μg/kg/min) + dobutamine (μg/kg/min) + 100 × epinephrine (μg/kg/min) + 100 × norepinephrine (μg/kg/min) + 50 × levosimendan (μg/kg/min) + 10,000 × vasopressin dose (U/kg/min) + 10 × milrinone dose (μg/kg/min) [13. ].
Subsequently, multivariable analyses with logistic regression were performed to investigate whether intra-LAC could be an independent factor for postoperative mortality. The following variables were selected based on previous reports: sex [14. ], age [15. ], surgery for intestinal ischemia/necrosis [16. ], pre-existing ischemic heart disease [17. ], preoperative Hb level [18. ], and sequential organ failure assessment (SOFA) score [6. ]. Intraoperative factors that could influence lactate levels were also selected as follows: maximum intraoperative VIS [13. , 19. ], total amount of intraoperative fluid administration [20. , 21. ], total amount of RBC transfusion, and hemorrhage [22. , 23. ]. SOFA score was calculated as a representative of the preoperative physical status [24. , 25. ].
Finally, the ability of intra-LAC to predict mortality was assessed using the area under the curve (AUC) determined from the receiver operating characteristic (ROC) curve and compared with post-LAC and LAC-C. The cutoff value of intra-LAC for postoperative mortality was calculated using the Youden index.
All statistical analyses were performed using EZR, a graphical user interface for R version 1.54 (R open source). More precisely, this is a modified version of the R commander designed to add statistical functions frequently used in biostatistics [26. ]. Continuous variables were reported as medians with interquartile ranges (IQRs) and compared using the non-parametric Mann–Whitney U test. Categorical variables were presented as frequencies (%) and were evaluated using Fisher’s exact test. The AUC of the ROC curves were compared using EZR statistical guide. Statistical significance was set at P < 0.05.
Results
We identified 551 emergency GI surgery cases that met our inclusion criteria over a 10-year observation period (Table 1). Overall, 120 patients died postoperatively, whereas 431 survived, with overall mortality rate of 21.8%.
Among the patients who died in-hospital there was a higher proportion of males compared with the patients who survived (64.0% vs. 48.3%, P = 0.002). The patients who died in hospital were older (79 [IQR, 72–84] vs. 71 [IQR, 61–79] years old, P < 0.001) than the survival group, and had a higher proportion of pre-existing ischemic heart disease (19.2% vs. 8.8%, P = 0.003), liver disease (4.2% vs. 0.7%, P = 0.014), and kidney disease (12.5% vs. 6.3%, P = 0.031). Patients who died in hospital had a lower proportion of surgery for upper GI perforation (11.7% vs. 20.9%, P = 0.025) and a higher proportion of surgery for intestinal ischemia/necrosis (37.5% vs. 9.7%, P < 0.001) compared with survivors. The patients who died had lower hemoglobin levels (10.7 [IQR, 9.1–13.1] g/dL vs. 12.4 [IQR, 10.6–14.4] g/dL, P < 0.001) and higher SOFA scores (8 [IQR, 5–11] vs. 2 [IQR, 1–5] points, P < 0.001). With regard to patient condition, the mortality group had a higher frequency of preoperative shock (61.7% vs. 18.6%, P < 0.001) and management with mechanical ventilation (50.0% vs. 14.8%, P < 0.001).
Table 2 presents a comparison of intraoperative hemodynamic management between the groups. The operative time did not differ significantly between the groups (patients who died vs. those who survived:137 [IQR 96–188] min vs. 135 [IQR 100–189] min, P = 0.887). The patients who died had higher volumes of hemorrhage (95 mL [IQR 2–802 mL] vs. 40 mL [IQR 0–254 mL], P = 0.001) and were administered higher volumes of fluid (4185 [IQR 2353 –6679] mL vs. 3200 [IQR 2200–4710], P = 0.002), RBC transfusion (4 [IQR, 0–6] units vs. 0 [IQR, 0–2] units, P < 0.001), and HES/colloid administration (225 [IQR, 0–500] units vs. 0 [IQR, 0–500] units, P = 0.044) than the patients who survived. Lower urine output (113 [IQR, 0–300] vs. 245 [IQR, 100–400] points, P < 0.001) was observed in the patients who died. The patients who died had a higher VIS (22 [IQR, 10–43] vs. 0 [IQR, 0–15] points, P < 0.001) than the patients who survived. The initial, intra-LAC and post-LAC levels were higher in the patients who died (3.46 [IQR, 1.84–6.26] vs. 1.56 [IQR, 1.03–2.64] mmol/L, P < 0.001, 4.22 [IQR, 2.15–7.13] vs. 1.80 [IQR, 1.19–3.01] mmol/L, P < 0.001 and 3.72 [IQR, 1.97–7.36] vs. 1.70 [IQR, 1.11–2.77] mmol/L, P < 0.001, respectively). The LAC-C rate was not significantly different between the groups (2.88 [IQR, -19.0–28.4] vs. 5.78 [IQR, -20.6–39.5], P = 0.796).
Multivariate analysis using logistic regression revealed that intra-LAC (odds ratio [OR] 1.21, 95% CI 1.07–1.36, P = 0.002) was an independent factor to predict in-hospital mortality after surgery (Table 3). Male sex, (OR 0.546, 95% CI 0.309–0.965, P = 0.037), older age (OR 1.050, 95% CI 1.020–1.070, P = 0.001), intestinal ischemia/necrosis (OR 2.700, 95% CI 1.370 –5.330, P = 0.004), preoperative hemoglobin level (OR 0.881, 95% CI 0.786–0.988, P = 0.030) and SOFA score (OR 1.230, 95% CI 1.130–1.340, P < 0.001) had statistically significant differences between the two groups of patients.
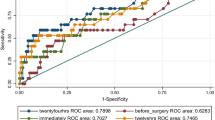
The AUCs of the initial-LAC, intra-LAC, post-LAC, and LAC-C for in-hospital mortality determined from the ROC curve analysis were as follows: AUC = 0.735, 95% CI, 0.682–0.789; AUC = 0.762, 95% CI, 0.711–0.812; AUC = 0.748, 95% CI, 0.695–0.801; and AUC = 0.508, 95% CI: 0.451–0.564, respectively (Fig. 2). The AUC of intra-LAC was larger than that of initial-LAC (P = 0.024) and LAC-C (P < 0.001) but did not differ from that of post-LAC (P = 0.306). The cutoff value of intra-LAC for postoperative mortality was 3.68 mmol/mL (sensitivity, 0.575; specificity, 0.833), calculated using the Youden index.
Predictive ability of intraoperative serum lactate levels for hospital mortality (N = 551). The AUC of intra-LAC was larger than that of the initial-LAC (P = 0.024) and not significantly different from that of post-LAC (p = 0.306). The cut-off values of initial LAC, intra-LACand post-LAC for postoperative mortality were 3.58 mmol/L (sensitivity, 0.5; specificity, 0.856), 3.68 mmol/L (sensitivity, 0.575; specificity, 0.833) and 3.33 mmol/L (sensitivity, 0.558; specificity, 0.821), respectively, Youden index. Initial LAC, initial lactate level; intra-LAC, intraoperative peak lactate level; post-LAC, postoperative lactate level; LAC-C, intraoperative lactate clearance; AUC, area under the curve
Discussion
In this study, we examined the prognostic value of intraoperative lactate level for outcomes after emergency GI surgery. The main finding of this study was that intraoperative hyperlactatemia was strongly associated with increased mortality after emergency GI surgery.
We performed a logistic regression analysis to investigate whether the intraoperative peak lactate level would be an independent predictor of prognosis. We found that intraoperative peak lactate level could be a prognostic factor for mortality. Several previously reported representative prognostic factors were selected for the multivariate analysis. As critically ill patients sometimes require a large amount of fluid to improve hemodynamic failure, fluid management during surgery can be associated with prognosis. Excessive fluid administration is a risk factor for fluid-related medical interventions, and a high central venous pressure is associated with poor prognosis [20. , 21. ]. Transfusion has also been associated with postoperative complications. Turan et al. reported that massive perioperative transfusion increases the risk of respiratory complications and infectious diseases [22. ]. Nacionales et al. reported that RBC transfusion alters the immune response during sepsis in mice, suggesting that transfusion may lead to poor outcomes in critically ill patients [27. ]. In contrast, the Transfusion Requirements in Septic Shock trial showed that lower and higher Hb thresholds for transfusion in septic shock did not influence mortality or the use of life support [28. ]. American Society of Anesthesiologists Task Force recommend RBC transfusion should be based on cardiopulmonary reserve as well [23. ]. Ischemic heart disease (IHD) is associated with perioperative cardiac events and mortality [17. ]. Intraoperative VIS could reportedly be a predictor of postoperative outcomes in cardiac surgery [13. , 19. ]. We also considered the preoperative condition presented in the severity scoring system, such as the SOFA score, which is an objective score obtained from the calculation of six organ dysfunctions (respiratory, coagulation, liver, cardiovascular, renal, and central nervous systems) [24. ]. Lactate levels can vary intraoperatively depending on various factors, such as the metabolic balance of organs and fluid balance during hemodynamic management. Interestingly, our logistic analysis showed that hemorrhage, amount of RBC transfusion and fluid administration, and VIS were not predictive of outcomes, showing the relevance of lactate measurement during surgery. The measurement of intraoperative lactate levels in patients may be useful as one of the intraoperative strategies.
Several reports have demonstrated the prognostic value of lactate levels in patients with acute gastrointestinal diseases. Kang et al. reported that the postoperative lactate level was a strong predictor of in-hospital mortality in patients who underwent surgery for GI perforation (AUC = 0.771) [29. ]. On the other hand, Jung et al. reported that lactate level (AUC = 0.659) measured in the emergency department in patients with Intra-abdominal infections had a lower predictive value for in-hospital mortality (AUC = 0.795) than SOFA score [6. ]. Our study showed that intraoperative lactate levels were not significantly different from postoperative lactate levels in predicting postoperative in-hospital mortality. Moreover, there was no significant difference between intraoperative lactate levels and SOFA scores (Supplemental Fig. 1). Although our study population was not the same as other studies, our findings suggest that intraoperative peak lactate level may help to predict prognosis.
In critically ill patients, absolute lactate levels and lactate clearance can predict patient outcomes. Haas et al. reported an association between 12-h lactate clearance in patients with severe hyperlactatemia and intensive care unit mortality [30. ]. Lokhandwala et al. reported that a > 20% reduction in lactate levels from baseline at 6 h was associated with in-hospital mortality [31. ]. In addition, the Surviving Sepsis Campaign guidelines of 2016 and 2021 recommend normalizing lactate levels as a therapeutic strategy [32. , 33. ]. However, the intraoperative lactate clearance calculated in our study population was not useful in predicting postoperative mortality. One possible explanation is that our study population included many patients with lactate levels within the normal range (< 2 mmol/L). Another possibility is that the operation time was too short to assess lactate clearance. Since lactate measurement following surgery was not possible in very critical patients because of their early death or other factors, we evaluated intraoperative lactate clearance using postoperative lactate levels in the present study; however, future studies should examine the relationship between perioperative lactate clearance and postoperative management.
Limitations
Our study has several limitations regarding the interpretation of the results. First, our single-center retrospective study had a small sample size. Second, the effects of confounding factors were not completely minimized in our analysis as we selected patients requiring emergency GI surgery. The complexity of the preoperative health status varied significantly between the cases, which would have influenced preoperative management. Time to surgery also reportedly influences the prognosis of patients with septic shock who require emergency GI surgery [34. ]. As this study included out-of-hospital-onset surgeries as well as in-hospital-onset surgeries (e.g., anastomotic leakage after scheduled GI surgery), the relationship between time-to-surgery and lactate levels could not be evaluated. Third, a lactate measurement protocol was not established in this retrospective study. The peak LACs were not the real peak levels because a continuous lactate monitoring device was not available [35. ]. Finally, the relationship between lactate levels and anesthetic agents was not investigated in this study. Since anesthetic agents can cause dose-related cardiovascular or hemodynamic depression, the dosage of anesthetic agents should be reduced as carefully as possible in patients with hemodynamic instability [36–42]. However, it was difficult to investigate how the anesthetic agents for each surgical stress affected lactate levels. In addition, preoperative sedatives or analgesics might influence intraoperative anesthesia. Patients with or without preoperative mechanical ventilation were included in this study, which might result in differences in the intraoperative dosage of anesthetic agents. Therefore, a well-designed prospective study is required to satisfactorily evaluate the relationship between lactate management and prognosis after emergency surgery.
Conclusions
In emergency GI surgery, the intraoperative lactate level, but not hemodynamic managements, was independently associated with increased in-hospital mortality. The prognostic value of intraoperative lactate level for in-hospital mortality was comparable to that of postoperative lactate level. Lactate measurement during surgery may be useful; however, the prognostic ability of lactate clearance during surgery was poor. Further studies are needed to investigate intraoperative strategies based on the lactate levels.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- GI:
-
Gastrointestinal
- Intra-LAC:
-
Intraoperative peak lactate level
- Initial-LAC:
-
Initial lactate level
- Post-LAC:
-
Postoperative lactate level
- LAC-C:
-
Intraoperative lactate clearance
- RBC:
-
Red blood cell
- Hb:
-
Hemoglobin
- VIS:
-
Vasoactive inotrope score
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SOFA:
-
Sequential organ failure assessment
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- IQR:
-
Interquartile range
References
Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2015;372:1078–9.
Saugel B, Trepte CJ, Heckel K, Wagner JY, Reuter DA. Hemodynamic management of septic shock: is it time for “individualized goal-directed hemodynamic therapy” and for specifically targeting the microcirculation? Shock. 2015;43:522–9.
Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, Reinhart K, Selvakumar N, Levy MM. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43:567–73.
Bou Chebl R, El Khuri C, Shami A, Rajha E, Faris N, Bachir R, Abou Dagher G. Serum lactate is an independent predictor of hospital mortality in critically ill patients in the emergency department: a retrospective study. Scand J Trauma Resusc Emerg Med. 2017;25:69.
Hajjar LA, Almeida JP, Fukushima JT, Rhodes A, Vincent JL, Osawa EA, Galas FR. High lactate levels are predictors of major complications after cardiac surgery. J Thorac Cardiovasc Surg. 2013;146:455–60.
Jung YT, Jeon J, Park JY, Kim MJ, Lee SH, Lee JG. Addition of lactic acid levels improves the accuracy of quick sequential organ failure assessment in predicting mortality in surgical patients with complicated intra-abdominal infections: a retrospective study. World J Emerg Surg. 2018;13:14.
Creagh-Brown BC, De Silva AP, Ferrando-Vivas P, Harrison DA. Relationship Between Peak Lactate and Patient Outcome Following High-Risk Gastrointestinal Surgery: Influence of the Nature of Their Surgery: Elective Versus Emergency. Crit Care Med. 2016;44:918–25.
Shimazaki J, Motohashi G, Nishida K, Ubukata H, Tabuchi T. Postoperative arterial blood lactate level as a mortality marker in patients with colorectal perforation. Int J Colorectal Dis. 2014;29:51–5.
Jimenez Rodriguez RM, Segura-Sampedro JJ, Flores-Cortés M, López-Bernal F, Martín C, Diaz VP, Ciuro FP, Ruiz JP. Laparoscopic approach in gastrointestinal emergencies. World J Gastroenterol. 2016;22:2701.
Navez B, Navez J. Laparoscopy in the acute abdomen. Best Pract Res Clin Gastroenterol. 2014;28:3–17.
Lupinacci RM, Menegaux F, Trésallet C. Emergency laparoscopy: Role and implementation. J Visc Surg. 2015;152:S65-71.
The Japan Society of Transfusion Medicine and Cell Therapy. Available from: http://yuketsu.jstmct.or.jp/en/
Koponen T, Karttunen J, Musialowicz T, Pietiläinen L, Uusaro A, Lahtinen P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122:428–36.
Peterson CY, Osen HB, Tran Cao HS, Yu PT, Chang DC. The battle of the sexes: women win out in gastrointestinal surgery. J Surg Res. 2011;170:e23-28.
Dowgiałło-Wnukiewicz N, Kozera P, Lech P, Rymkiewicz P, Michalik M. Emergency surgery in older patients. Videosurgery Other Miniinvasive Techniques. 2019;14:182–6.
Bala M, Catena F, Kashuk J, De Simone B, Gomes CA, Weber D, et al. Acute mesenteric ischemia: updated guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2022;17:54.
Cao D, Chandiramani R, Capodanno D, Berger JS, Levin MA, Hawn MT, Angiolillo DJ, Mehran R. Non-cardiac surgery in patients with coronary artery disease: risk evaluation and periprocedural management. Nat Rev Cardiol. 2021;18:37–57.
Boyd-Carson H, Shah A, Sugavanam A, Reid J, Stanworth SJ, Oliver CM. The association of pre-operative anaemia with morbidity and mortality after emergency laparotomy. Anaesthesia. 2020;75:904–12.
Yamazaki Y, Oba K, Matsui Y, Morimoto Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth. 2018;32:167–73.
Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43:68–73.
Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014;4:21.
Turan A, Yang D, Bonilla A, Shiba A, Sessler DI, Saager L, Kurz A. Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can J Anaesth. 2013;60:761–70.
American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology. 2015;122:241–75.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Nacionales DC, Cuenca AG, Ungaro R, Gentile LF, Joiner D, Satoh M, Lomas-Neira J, Ayala A, Bihorac A, Delano MJ, et al. The acute immunological response to blood transfusion is influenced by polymicrobial sepsis. Shock. 2012;38:598–606.
Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Åneman A, Vang ML, Winding R, et al. Lower versus Higher Hemoglobin Threshold for Transfusion in Septic Shock. N Engl J Med. 2014;371:1381–91.
Kang MK, Oh SY, Lee H, Ryu HG. Pre and postoperative lactate levels and lactate clearance in predicting in-hospital mortality after surgery for gastrointestinal perforation. BMC Surg. 2022;22:93.
Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, Kluge S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. 2016;42:202–10.
Lokhandwala S, Andersen LW, Nair S, Patel P, Cocchi MN, Donnino MW. Absolute lactate value vs relative reduction as a predictor of mortality in severe sepsis and septic shock. J Crit Care. 2017;37:179–84.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304–77.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–247.
Azuhata T, Kinoshita K, Kawano D, Komatsu T, Sakurai A, Chiba Y, Tanjho K. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 2014;18:R87.
Ming DK, Jangam S, Gowers SAN, Wilson R, Freeman DME, Boutelle MG, Cass AEG, O’Hare D, Holmes AH. Real-time continuous measurement of lactate through a minimally invasive microneedle patch: a phase I clinical study. BMJ Innov. 2022;8:87–94.
Ebert TJ, Harkin CP, Muzi M. Cardiovascular responses to sevoflurane: a review. Anesth Analg. 1995;81:S11–22.
Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41.
Keating GM. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs. 2015;75:1119–1130.
Khademi H, Kamangar F, Brennan P, Malekzadeh R. Opioid Therapy and its Side Effects: A Review. Arch Iran Med. 2016;19:870–6.
Goren O, Matot I. Perioperative acute kidney injury. Br J Anaesth. 2015;115:ii3–14.
Schenk J, Wijnberge M, Maaskant JM, Hollmann MW, Hol L, Immink RV, et al. Effect of Hypotension Prediction Index-guided intraoperative haemodynamic care on depth and duration of postoperative hypotension: a sub-study of the Hypotension Prediction trial. Br J Anaesth. 2021;127:681–8.
Weinberg L, Li SY, Louis M, Karp J, Poci N, Carp BS, et al. Reported definitions of intraoperative hypotension in adults undergoing non-cardiac surgery under general anaesthesia: a review. BMC Anesthesiol. 2022;22:69.
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Funding
This study was not supported by any funding agency.
Author information
Authors and Affiliations
Contributions
Shinji Sugita: Conceptualization, methodology, investigation, data curation, formal analysis, validation, visualization, supervision, and writing–original draft. Masashi Ishikawa: methodology, formal analysis, and validation. Takahiro Sakuma: investigation, formal analysis, validation, and visualization. Masumi Iizuka: investigation, formal analysis, validation, and visualization. Sayako Hanai: investigation, formal analysis, validation, and visualization. Atsuhiro Sakamoto: conceptualization, methodology, formal analysis, validation, and project administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participation
This study was approved by the ethics committee of the Nippon Medical School (no. 26–02-427). All experiments were performed in accordance with the government ethical guidelines and regulations based on the Declaration of Helsinki. The need for informed consent for each participant was waived by the ethics committee of Nippon Medical School due to retrospective nature of the study and the form of an opt-out option (guarantee of information disclosure and opportunity to refuse) was provided on our institution’s website.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplement Figure 1. Prediction ability of SOFA score for hospital mortality. The AUC of the SOFA score for postoperative mortality was not significantly different from that of intra-LAC but was larger than that of initial LACand post-LAC . The cut-off value of the SOFA score for postoperative mortality was 7 points, calculated using the Youden index. SOFA, sequential organ failure assessment; AUC, area under the curve; intra-LAC, intraoperative peak lactate level; initial-LAC, initial lactate level; post-LAC, postoperative lactate level.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sugita, S., Ishikawa, M., Sakuma, T. et al. Intraoperative serum lactate levels as a prognostic predictor of outcome for emergency abdominal surgery: a retrospective study. BMC Surg 23, 162 (2023). https://doi.org/10.1186/s12893-023-02075-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-023-02075-7