Abstract
Background
With increasing use and enhanced accuracy of cross-sectional imaging, the diagnosis of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas has increased over the last few decades. The extent to which malignant transformation occurs remains unclear, making the management of IPMNs controversial. The aim of this study was to evaluate the progression rate and outcome of follow-up in patients with IPMNs.
Methods
A database of all patients diagnosed with IPMN at the Cantonal Hospital HFR Fribourg, Switzerland, between January 2006 and December 2019 with a follow-up of at least 6 months was analyzed retrospectively. Descriptive statistics were performed on patient demographics, IPMN characteristics, and follow-up data.
Results
A total of 56 patients were included in this study. Ten patients underwent primary surgery, 46 were enrolled in a surveillance program.21.7% (n = 5) of patients under surveillance presented with worrisome features of IPMN; progression rates were significantly higher in these patients (p = 0.043). Most progression occurred in the early follow-up period. Five patients underwent surgery due to progression, of which 2 presented high-grade dysplasia and 2 malignancy on postoperative histology.
Conclusions
The limited predictive value of current guidelines may lead to surgical overtreatment, and the decision to proceed with surgical resection should be made with caution. Further prospective analyses and the development of novel biomarkers are needed to better understand the natural history of IPMN and improve diagnostic precision.
Similar content being viewed by others
Introduction
With increasing use and enhanced accuracy of computed tomography (CT) and magnetic resonance imaging (MRI), the incidental detection of intraductal papillary mucinous neoplasms (IPMNs) of the pancreas has increased over the last few decades. Up to 15% of patients undergoing abdominal MRI are diagnosed with a cystic lesion, which may be an IPMN in up to 82% of cases [1].
IPMNs of the pancreas are mucous-producing, cystic tumors originating from the pancreatic epithelium of the main pancreatic duct (MPD) or its side branches. Accordingly, they are classified into three groups [main duct (MD), branch duct (BD), or mixed type (MT) IPMN] and can evolve as unifocal or multifocal lesions. IPMN can be considered benign, borderline, or malignant depending on the grade of dysplasia [2,3,4]. Even though such lesions should always be considered as precursor lesions for pancreatic ductal adenocarcinoma (PDAC), it remains unclear the proportion to which malignant transformation occurs, with reported rates varying between 18 and 25% for BD-IPMN and 60% and 70% for MD-IPMN [5, 6]. To avoid malignant transformation, surgical resection remains the treatment of choice. However, the morbidity inherent with pancreatic surgery is not negligible and, as many IPMNs will never progress to malignancy, unnecessary surgery should be avoided [5, 6].
Due to the lack of knowledge regarding the natural history of these lesions, the management of IPMN remains controversial. Several guidelines based on expert opinions and retrospective studies have been established to predict the malignant potential and provide guidelines for follow-up and surgical decision-making 3, 4, 7–9. Guidelines developed by the International Association of Pancreatology [7] and the European guidelines from the European Study Group on Cystic Tumours of the Pancreas [8] are both widely used and identify obstructive jaundice, the presence of an enhancing mural nodule ≥ 5 mm or a solid cyst component, positive cytology for high-grade dysplasia (HGD) or invasive cancer, and a dilated MPD ≥ 10 mm as highly predictive of advanced neoplasia in IPMN and an indication for resection in surgically fit patients [9]. Further investigations, such as endoscopic ultrasound, should be performed in the presence of worrisome features [WFs; recurrent pancreatitis, cyst size ≥ 3 cm, cyst growth rate of ≥ 5 mm/2 years, thickened or enhancing cyst walls, MPD ≥ 5 mm, abrupt change in MPD caliber with distal pancreatic atrophy, and lymphadenopathy or increased serum level of carbohydrate antigen (CA19-9)], which are considered indications for surgery. Especially for MD- and MT-IPMN, a high index of suspicion is warranted due to higher risk of malignant transformation compared to BD-IPMN. For most patients with BD-IPMN, surveillance programs with periodic MRI every 6 to 24 months are recommended, with the interval of surveillance depending on the size of the largest cyst [3, 7, 8].
Current guidelines for the management of IPMN are based on limited evidence, and the safety, method, and duration of surveillance are still highly debated. Therefore, the aim of the present study was to analyze the progression rates and management strategies in all patients with IPMN who entered a surveillance program or were treated surgically at our center.
Material and methods
Study procedures
We conducted a retrospective observational study of patients presenting with an IPMN of the pancreas who were under surveillance or treated in the surgical clinic at the Cantonal Hospital HFR Fribourg, Switzerland. This study was approved by the cantonal Ethics Committee (Project-ID 2020-00332, Ethics Committee Vaud) and was conducted in accordance with the guidelines and regulations of the Declaration of Helsinki. The data were studied following the STROBE guidelines [10].
Patient eligibility
We included all patients diagnosed with an IPMN of the pancreas within the surgical clinic at the Cantonal Hospital Fribourg, Switzerland, between January 2006 and December 2019. At the time of diagnosis, patients were either planned for primary surgical resection or enrolled in a surveillance program. Exclusion criteria were follow-up shorter than 6 months in patients enrolled in the surveillance program and misdiagnosis established radiologically during surveillance or histologically in the surgical specimen. Cases in which the diagnosis changed after initial diagnosis of IPMN were excluded in order to minimize selection bias. Informed consent was provided by all study participants.
Data collection
All data were collected from the patient’s medical records. Random study ID allocation was generated via Excel® and data extraction performed using REDcap®. Extracted data included demographic data (age, sex, and comorbidities), radiographic reports from MRI, CT, endosonography (EUS) with or without fine-needle aspiration biopsy (FNA), and ultrasound, surgical and other physician consultations (gastroenterology, emergency room, and general practitioner), operative reports, and histological and cytological reports.
Definitions
Patients were defined as being enrolled in the surveillance program if there was no surgical resection performed at time of diagnosis after the initial work-up. The surveillance program is supervised and led by the head pancreatic surgeon of our tertiary center and consists of a regular clinical and radiological follow-up according to current guidelines [3, 7, 8]. Follow-up duration was recorded as time in months between the initial IPMN diagnosis on first cross-sectional imaging and last available imaging data. WFs and high-risk stigmata (HRS) were defined according to criteria from current guidelines [3, 7, 8]. IPMN progression was defined as the appearance of at least one WF during follow-up.
Statistical analysis
Medians with interquartile ranges (IQR), or percentages were calculated for the overall sample and subgroups. Descriptive statistics were computed for the patient’s demographics, mode of detection, and IPMN characteristics at diagnosis, as well as follow-up data and progression rates. Dichotomous data were reported as the number and proportions, continuous data as medians with interquartile range. Progression-free survival was estimated using the Kaplan–Meier method. Progression-free survival between the subgroups was compared by the Log rank test. All statistical analyses were performed using IBM® SPSS® Statistics 26. A two-sided level of significance of 0.05 was used for all analyses.
Results
Patient characteristics
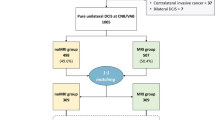
Figure 1 illustrates patient selection. Between 2006 and 2019, 73 patients were diagnosed with an IPMN at our center. Among them, 13 were planned for primary resection, 3 of which were excluded because histological analysis of the surgical specimen concluded in another diagnosis (1 neuroendocrine tumor of the pancreas and 2 serous cystadenoma). Among the 60 patients initially selected for a surveillance program, 3 were excluded as diagnosis of IPMN was discarded during follow-up (1 pseudocyst diagnosed by EUS-FNA, 1 chronic pancreatitis with pseudocysts, and 1 serous cystadenoma on surgical specimen after secondary surgical resection) and 11 were excluded due to follow-up shorter than 6 months. We finally included 56 patients presenting with an IPMN of the pancreas at our center between 2006 and 2019, 10 of whom underwent primary surgery and 46 were enrolled in the surveillance program. Table 1 describes the characteristics of the study participants at diagnosis; 50.0% (n = 28) of the included patients were women, and the median age at diagnosis was 68 years (IQR = 9.5). Among the included patients, 16.1% (n = 9) presented with chronic pancreatitis and 14.3% (n = 8) with diabetes as a comorbidity. IPMN was diagnosed by abdominal CT in 82.2% (n = 46) of cases. In 21.4% (n = 12) of patients, abdominal pain or further imaging in the context of acute pancreatitis led to the diagnosis of IPMN. However, in most patients, IPMN was an incidental finding on imaging (78.6%, n = 44)). Diagnosis was then confirmed by MRI and / or EUS in all patients but three (93.5%, n = 43). These three patients did regular follow-up by CT due to colon or head and neck cancer.
The most frequent IPMN types at diagnosis were BD-IPMN (73.2%, n = 41), followed by MD-IPMN (16.1%, n = 9) and MT-IPMN (10.7%, n = 6); 39.3% (n = 22) of the IPMNs were localized in the head of the pancreas, 26.8% (n = 15) in the body, 25.0% (n = 14) in the uncinated process, 12.3% (n = 8) in the neck, and 16.1% (n = 9) in the pancreatic tail. Median cyst size at diagnosis was 16 mm (IQR = 13). Overall, 26.8% (n = 15) of patients presented with WFs at diagnosis, including 9 patients diagnosed with cyst ≥ 3 cm. Three patients presented with HRS at diagnosis (5.6%) and underwent primary surgery without previous enrollment in the surveillance program.
Surveillance program
Forty-six patients were enrolled in the surveillance program (Table 2).
Patients with WF at diagnosis (WF +)
Ten patients presented with an IPMN with WFs at diagnosis (21.7%). The median follow-up time was 21 months (IQR = 12.8) with a median time to progression of 16 months (IQR = 5). Half of the patients underwent diagnostic EUS-FNA, and further EUS-FNA was performed during follow-up in 60% (n = 6) of the cases. Of all patients with WF + IPMN, 50% (n = 5) showed progression during surveillance and 20% (n = 2) underwent surgery.
Eight patients had a single WF (cyst size ≥ 3 cm, MPD dilatation ≥ 5 mm or enhancing cyst walls). Three of these patients exhibited progression after 10, 16, and 19 months: One patient underwent surgery (#12 in Table 3), one patient decided to pursue surveillance, and one patient refused further investigations and surveillance. Two patients presented with more than one WF. In the first case, explorative laparotomy with the objective of primary resection by partial pancreatectomy was performed in a 70-year-old female patient 9 months after first diagnosis of a multifocal MT-IPMN of the entire pancreas with a maximum cyst size of 36 mm and MPD dilatation of 11 mm. As the patient refused total pancreatectomy, which was indicated due to the extent of the disease, resection was not performed and she was enrolled in the surveillance program. Cyst growth of 9 mm/2 years (to 45 mm) was noted during 71 months of surveillance, and then disease stability for another 50 months, bringing the total follow-up duration up to 121 months. In a second case, a 50-year-old male with MT-IPMN 84 mm in size with MPD dilatation of 8 mm was enrolled in the surveillance program due to normal results on cytology and normal serum CA19-9 levels. However, due to progression after 14 months (cyst size 100 mm), the patient (#11 in Table 3) underwent pancreaticoduodenectomy 23 months after diagnosis, revealing HGD.
Patients without WF at diagnosis (WF−)
Thirty-six (78.2%) patients in the surveillance program did not exhibit WFs at diagnosis. Six of these patients presented progression during follow-up (16.7%), with a median time to progression of 9 months (IQR = 4). The median follow-up time was 23 months (IQR = 27.5). Eight patients (22.2%) underwent diagnostic EUS-FNA and further EUS-FNA was performed during the course of surveillance in 30.6% of the cases.
One patient (#13 in Table 3) underwent surgery after 10 months of follow-up due to rapid cyst growth in a young, fit, male patient with BD-IPMN (see Table 3). Two patients presented with concomitant disease, which led to the indication for surgery (serous cystadenoma of the pancreas in patient #14 and gastric GIST in patient #15, which was also treated by gastric wedge resection during the intervention).
Overall progression
Of the 46 patients who entered surveillance, 5 underwent surgical resection. Progression during follow-up led to surgical management in three of the patients, and concomitant disease led to surgery in two of the patients (see Fig. 2).
Figure 3 shows the progression-free survival for patients enrolled in the surveillance program. Most new WFs appeared during early follow-up, but some progression occurred after up to 77 months. Median time to progression in patient with WFs at diagnosis (WF +) was 16 months (IQR = 5) versus 9 months (IQR = 4) in patients presenting with no WF at diagnosis (WF-). There was a significantly higher rate of progression-free survival during follow-up in WF- patients compared to WF + patients (p = 0.043).
In two patients, surveillance was stopped due to disease stability after 100 months or regression after 6 months. Overall, nine patients did not wish to continue follow-up (19.6%) and three of the patients (6.5%) withdrew from surveillance due to comorbidities and malignancies or death not related to IPMN.
Primary surgical management
As shown in Table 3, 10 of the 56 included patients underwent primary surgical resection (17.9%). In three cases, patients presented with HRS (either HGD on cytology or obstructive jaundice) at diagnosis. In five cases, the indication for primary surgery was the presence of at least two WFs at diagnosis or one WF in MT-IPMN. One patient underwent surgical resection because of a concomitant neuroendocrine tumor of the pancreas and one patient due to elevated carcinoembryonic antigen (CEA) in the cyst fluid suspicious of mucinous cystadenoma. See Table 3 for details on surgically managed patients.
Cytological and histological analyses
Preoperative EUS-FNA was performed in all patients undergoing surgical resection. Post-operative histological analysis of all patients undergoing surgery showed low-grade dysplasia in most patients (53.3%, n = 8). In the 10 patients undergoing primary surgery, 2 exhibited HGD preoperatively, but it was not confirmed on post-operative histological analysis. Malignant transformation was diagnosed in two patients who underwent primary surgery but did not exhibit HGD on preoperative cytology. In the five patients enrolled in the surveillance program, histological analysis of the surgical specimen found HGD in two cases but no cases of malignant transformation. Table 3 provides the details of the patients who underwent surgical resection.
Surgical morbidity and mortality
Among the 15 operated patients, postoperative morbidity within 30 days after surgery was 33% (n = 5), with 2 presenting a complication grade II, 2 presenting a grade IIIa, and 1 patient presenting a grade IIIb according to Clavien-Dindo Classification [11]. At 1-year follow-up, postoperative mortality was found to be 0%. One patient (#5 in Table 3) died 16 months after surgery of a cause unrelated to surgery and one (#13 in Table 3) 7 years later of pancreatic adenocarcinoma that developed after 5 years of uneventful follow-up.
Discussion
The primary goal in the management of patients with IPMN is to prevent progression to malignancy while avoiding unnecessary surgery. However, due to a lack of knowledge about the rate and timing of malignant progression, the management of this entity remains challenging even though the diagnosis of IPMN of the pancreas is increasing due to the increased use of advanced imaging [9, 12].
In accordance with the literature, our analyses showed that IPMNs are often an incidental finding, with at least 78.6% of patients in our series being asymptomatic. In patients enrolled in the surveillance program, 21.7% presented with WFs at diagnosis. In our series, these patients were shown to be significantly more likely to progress, suggesting the need for shorter follow-up intervals. Most patients showed progression early during surveillance but, in some cases, new WFs appeared after more than 5 years of disease stability. Accordingly, several authors have reported long-lasting risk of the development of concomitant PDAC in patients with IPMN, and even the risk of developing new IPMN after resection in up to 62% at 10 years, with potential malignant progression [1, 7, 13,14,15,16]. Therefore, indefinite surveillance is suggested, even after surgical resection of IPMN. However, some authors have recommend the discontinuation of follow-up after 2–5 years of disease stability due to the small overall risk of malignant transformation, which outweighs the cost of surveillance and the psychological burden of disease [17,18,19]. The required duration of surveillance remains debatable. In our series, 19.6% of the patients discontinued surveillance on their own initiative, suggesting significant psychological burden. The potential benefit from longer surveillance needs to be balanced with psychological burden and cost-effectiveness, as well as the potential benefit from surgical resection.
Surgery is widely accepted as the only curative treatment for IPMN. However, the morbidity and mortality associated with pancreatic surgery is high, with post-operative morbidity of up to 30–50% [20], and needs to be cautiously balanced with the potential risk of malignant transformation. The malignant potential of IPMN remains uncertain, ranging from 0 to 32%, with an estimated average annual risk of 0.24% in absence of HRS [17, 21] and differing between the different types of IPMNs, with increased risk in MD- and MT-IPMN compared to BD-IPMN [3, 7, 22, 23]. Different research groups have attempted to define WFs to predict the risk of malignancy [4, 7, 13, 17,18,19, 21, 24,25,26,27]. Some authors have described cyst size as the main predictor, increasing the risk of malignant transformation threefold in the presence of a cyst larger than 3 cm, and eightfold if a solid component is associated [4, 21, 28, 29]. Other authors have suggested that the presence of enhancing mural nodules is highly suspicious for malignant transformation [30,31,32,33]. Cytological analyses and serum tumor markers have been described as helpful tools in the risk-stratification of cystic lesions [3, 19, 24, 25, 32], but some authors have found no evidence to suggest this as a routine diagnostic method [26, 34]. In our study, cytological analyses by EUS-FNA were performed in all patients undergoing surgery and in 37% of the patients during surveillance. HGD was found in two patients who then underwent primary surgery. However, both surgical specimens only showed low-grade dysplasia. On the other hand, patients with HGD or malignancy in the surgical specimen did not present with HGD in the cyst fluid analysis, suggesting low predictive power as a single predictor.
Even though the different high-risk features seem to be associated with progression and malignant transformation, these features alone have a relatively poor positive predictive value, ranging from 25 to 62%, which may result in surgical overtreatment [4, 16, 30, 32, 35,36,37,38]. In our study, 12 patients underwent surgical resection for the presence of HRS or progression of WF. Of these patients, 2 presented HGD and 2 PDAC. This is in accordance with a recent series, which showed 10% of PDAC and 20% of HGD in patients after pancreatectomy for IPMN [38].
Definitive diagnosis can only be established pathologically in patients who have undergone resection. In our cohort, five patients underwent surgery for preoperative diagnosis of IPMN, which was not later confirmed to be an IPMN, but another cystic lesion of the pancreas, such as serous cystadenoma. In patients entering surveillance programs without surgical resection, IPMNs are diagnosed radiologically, which could lead to a significant amount of misdiagnosis of both presumed IPMN and the presumed absence of associated malignancy. It is impossible to evaluate the actual incidence of malignancy in the overall population of individuals harboring high-risk IPMNs.
New predictive factors are needed to more accurately predict malignancy and improve surgical decision-making. Recent studies have shown promising results with the analysis of cyst fluids [39]. IPMNs with low-grade dysplasia and HGD have distinct molecular features with, for example, different levels of prostaglandin E synthase 2 or interleukin 1β, which could be indicators of malignant progression and, therefore, serve as biomarkers to identify patients with high-risk IPMNs [40,41,42]. Furthermore, mutational analysis of driver genes, such as KRAS, GNAS, or KLF4, as well as miRNA sequencing and cyst fluid telomere fusion status seem to allow the discrimination of high-risk IPMN from low risk lesions [43,44,45,46,47]. Panel analyses combining several markers have shown to further increase the specificity and sensitivity to accurately predict high malignant potential [48,49,50].
Currently, the management of IPMNs is still highly debated, and risk factors for IPMN need to be more clearly defined according to molecular, radiological, and clinical data. Our study gives insights into IPMN follow-up and associated problems with risk stratification for these lesions. There are some limitations to this study, namely the small sample size and lack of information on the post-operative follow-up of our patients. Furthermore, not all IPMNs included in the analysis were histologically proven because they were not all resected. There is a clear need for prospective (multi-institutional) studies of the long-term follow-up of IPMNs and the analysis of both molecular and clinical data.
Conclusion
In conclusion, IPMNs are a frequent entity with increasing incidence. Evidence-based guidelines regarding clinical management are lacking. Even though the presence of worrisome features seems to be associated with higher risk of progression, its prediction for the risk of malignancy remains uncertain. The limited positive predictive value of current guidelines may lead to surgical overtreatment, and the decision to intervene surgically should be made with precaution. Clinical decision-making should be based on the estimated risk of malignant transformation, patient’s age, comorbidities, and life circumstances. Further prospective analyses and the development of novel biomarkers are needed to better understand the natural history of IPMNs and improve diagnostic precision. In the meantime, the complexity of the management of IPMN should not be underestimated and should be centralized in centers with high volume and expertise.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CT:
-
Computed tomography
- IPMN:
-
Intraductal papillary mucinous neoplasms
- MPD:
-
Main pancreatic duct
- MD:
-
Main duct
- BD:
-
Branch duct
- MT:
-
Mixed type
- PDAC:
-
Pancreatic ductal adenocarcinoma
- CA 19-9:
-
Carbohydrate antigen
- HGD:
-
High-grade dysplasia
- WF:
-
Worrisome feature
- EUS:
-
Endosonography
- FNA:
-
Fine-needle aspiration biopsy
- HRS:
-
High-risk stigmata
- CEA:
-
Carcinoembryonic antigen
- IQR:
-
Interquartile range
References
Cortegoso Valdivia P, Chialà C, Venezia L, Gaiani F, Leandro G, Di Mario F, et al. Diagnosis and management of intraductal papillary mucinous neoplasms of the pancreas. Acta Bio Medica Atenei Parm. 2018;89:147–52.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–8.
Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang J-Y, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97.
Buscail E, Cauvin T, Fernandez B, Buscail C, Marty M, Lapuyade B, et al. Intraductal papillary mucinous neoplasms of the pancreas and European guidelines: importance of the surgery type in the decision-making process. BMC Surg. 2019;19:115.
Daudé M. Outcomes of nonresected main-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastroenterol. 2015;21:2658.
Malleo G, Marchegiani G, Borin A, Capelli P, Accordini F, Butturini G, et al. Observational study of the incidence of pancreatic and extrapancreatic malignancies during surveillance of patients with branch-duct intraductal papillary mucinous neoplasm. Ann Surg. 2015;261:984–90.
Tanaka M, Fernández-del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–53.
The European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804.
van Huijgevoort NCM, del Chiaro M, Wolfgang CL, van Hooft JE, Besselink MG. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16:676–89.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9.
Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Crippa S, Arcidiacono PG, De Cobelli F, Falconi M. Review of the diagnosis and management of intraductal papillary mucinous neoplasms. United Eur Gastroenterol J. 2020;8:249–55.
Tanaka M. Clinical management and surgical decision-making of IPMN of the pancreas. In: Su GH, editor. Pancreatic cancer. New York: Springer, New York; 2019. p. 9–22.
Miyasaka Y, Ohtsuka T, Tamura K, Mori Y, Shindo K, Yamada D, et al. Predictive factors for the metachronous development of high-risk lesions in the remnant pancreas after partial pancreatectomy for intraductal papillary mucinous neoplasm. Ann Surg. 2016;263:1180–7.
He J, Cameron JL, Ahuja N, Makary MA, Hirose K, Choti MA, et al. Is it necessary to follow patients after resection of a benign pancreatic intraductal papillary mucinous neoplasm? J Am Coll Surg. 2013;216:657–65.
Kang MJ, Jang J-Y, Lee KB, Chang YR, Kwon W, Kim S-W. Long-term prospective cohort study of patients undergoing pancreatectomy for intraductal papillary mucinous neoplasm of the pancreas: implications for postoperative surveillance. Ann Surg. 2014;260:356–63.
Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824-848.e22.
Sahani DV, Kambadakone A, Macari M, Takahashi N, Chari S, Castillo CF. Diagnosis and management of cystic pancreatic lesions. Am J Roentgenol. 2013;200:343–54.
Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754–73.
La Torre M, Ramacciato G, Nigri G, Balducci G, Cavallini M, Rossi M, et al. Post-operative morbidity and mortality in pancreatic surgery. The role of surgical Apgar score. Pancreatology. 2013;13:175–9.
Vege SS, Ziring B, Jain R, Moayyedi P, Adams MA, Dorn SD, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22.
Tanaka M, Chari S, Adsay V, Carlos Castillo F-D, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32.
Schellhaas B, Vitali F, Wildner D, Görtz RS, Pfeifer L, Konturek PC, et al. Dynamics of fukuoka criteria and patient management in pancreatic intraductal papillary mucinous neoplasms (IPMNs) during follow-up. Med Sci Monit. 2017;23:1483–92.
Jacobson BC, Baron TH, Adler DG, Davila RE, Egan J, Hirota WK, et al. ASGE guideline: the role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc. 2005;61:363–70.
Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339–49.
Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703–11.
Buscarini E, Pezzilli R, Cannizzaro R, Angelis CD, Gion M, Morana G, et al. Italian consensus guidelines for the diagnostic work-up and follow-up of cystic pancreatic neoplasms. Dig Liver Dis. 2014;46:479–93.
Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:913–21.
Nguyen AH, Toste PA, Farrell JJ, Clerkin BM, Williams J, Muthusamy VR, et al. Current recommendations for surveillance and surgery of intraductal papillary mucinous neoplasms may overlook some patients with cancer. J Gastrointest Surg. 2015;19:258–65.
Jang J-Y, Park T, Lee S, Kang MJ, Lee SY, Lee KB, et al. Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg. 2014;101:686–92.
Kim KW, Park SH, Pyo J, Yoon SH, Byun JH, Lee M-G, et al. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Ann Surg. 2014;259:72–81.
Ricci C, Casadei R, Taffurelli G, Zani E, Pagano N, Pacilio CA, et al. Risk factors for malignancy of branch-duct intraductal papillary mucinous neoplasms: a critical evaluation of the fukuoka guidelines with a systematic review and meta-analysis. Pancreas. 2016;45:1243–54.
Sultana A, Jackson R, Tim G, Bostock E, Psarelli EE, Cox TF, et al. What is the best way to identify malignant transformation within pancreatic IPMN: a systematic review and meta-analyses. Clin Transl Gastroenterol. 2015;6: e130.
Kucera S, Centeno BA, Springett G, Malafa MP, Chen YA, Weber J, et al. Cyst fluid carcinoembryonic antigen level is not predictive of invasive cancer in patients with intraductal papillary mucinous neoplasm of the pancreas. JOP J Pancreas. 2012;13:409–13.
Goh BKP, Lin Z, Tan DMY, Thng C-H, Khor CJL, Lim TKH, et al. Evaluation of the Fukuoka Consensus Guidelines for intraductal papillary mucinous neoplasms of the pancreas: Results from a systematic review of 1,382 surgically resected patients. Surgery. 2015;158:1192–202.
Watanabe Y, Nishihara K, Niina Y, Abe Y, Amaike T, Kibe S, et al. Validity of the management strategy for intraductal papillary mucinous neoplasm advocated by the international consensus guidelines 2012: a retrospective review. Surg Today. 2016;46:1045–52.
Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466–75.
Litchinko A, Kobayashi K, Halkic N. A retrospective study of histological outcome for IPMN after surgery in Lausanne, Switzerland: a case series. Ann Med Surg. 2020;60:110–4.
Hao S, Takahashi C, Snyder RA, Parikh AA. Stratifying intraductal papillary mucinous neoplasms by cyst fluid analysis: present and future. Int J Mol Sci. 2020;21:1147.
Schmidt CM, Yip-Schneider MT, Ralstin MC, Wentz S, DeWitt J, Sherman S, et al. PGE2 in pancreatic cyst fluid helps differentiate IPMN from MCN and predict IPMN dysplasia. J Gastrointest Surg. 2008;12:243–9.
DiMaio CJ, Weis-Garcia F, Bagiella E, Tang LH, Allen PJ. Pancreatic cyst fluid concentration of high-mobility group A2 protein acts as a differential biomarker of dysplasia in intraductal papillary mucinous neoplasm. Gastrointest Endosc. 2016;83:1205–9.
Maker AV, Katabi N, Qin L-X, Klimstra DS, Schattner M, Brennan MF, et al. Cyst fluid interleukin-1β (IL1β) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2011;17:1502–8.
Volckmar A-L, Endris V, Gaida MM, Leichsenring J, Stögbauer F, Allgäuer M, et al. Next generation sequencing of the cellular and liquid fraction of pancreatic cyst fluid supports discrimination of IPMN from pseudocysts and reveals cases with multiple mutated driver clones: first findings from the prospective ZYSTEUS biomarker study. Genes Chromosomes Cancer. 2019;58:3–11.
Fujikura K, Hosoda W, Felsenstein M, Song Q, Reiter JG, Zheng L, et al. Multiregion whole-exome sequencing of intraductal papillary mucinous neoplasms reveals frequent somatic KLF4 mutations predominantly in low-grade regions. Gut. 2021;70:928–39.
Matthaei H, Wylie D, Lloyd MB, Dal Molin M, Kemppainen J, Mayo SC, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–24.
Wang J, Paris PL, Chen J, Ngo V, Yao H, Frazier ML, et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015;356:404–9.
Hata T, Dal Molin M, McGregor-Das A, Song TJ, Wolfgang C, Eshleman JR, et al. Simple detection of telomere fusions in pancreatic cancer, intraductal papillary mucinous neoplasm, and pancreatic cyst fluid. J Mol Diagn. 2018;20:46–55.
Maker AV, Hu V, Kadkol SS, Hong L, Brugge W, Winter J, et al. Cyst fluid biosignature to predict intraductal papillary mucinous neoplasms of the pancreas with high malignant potential. J Am Coll Surg. 2019;228:721–9.
Simpson RE, Yip-Schneider MT, Flick KF, Wu H, Colgate CL, Schmidt CM. Pancreatic fluid interleukin-1β complements prostaglandin E2 and serum carbohydrate antigen 19–9 in prediction of intraductal papillary mucinous neoplasm dysplasia. Pancreas. 2019;48:1026–31.
Roth S, Bose P, Alhamdani MSS, Mustafa SA, Tjaden C, Zamzow K, et al. Noninvasive discrimination of low and high-risk pancreatic intraductal papillary mucinous neoplasms. Ann Surg. 2021;273:e273–5.
Tol JAMG, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156:591–600.
Acknowledgements
Not applicable.
Funding
The authors did not receive financial support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
BE conceived of the presented idea. All authors participated in the conception and design of the paper. SP and OB performed the data acquisition, analysis, and interpretation. The manuscript was drafted by SP and then critically revised by OB and BE. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research project was conducted in accordance with the guidelines and regulations of the Declaration of Helsinki and has been approved by the responsible cantonal Ethics Committee (Ethics Committee Vaud, www.cer-vd.ch), which is member of the Swiss Association of Research Ethics Committees (www.swissethics.ch/en). The project-ID is 2020-00332. Informed consent was obtained from all study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Peisl, S., Burckhardt, O. & Egger, B. Limitations and prospects in the management of IPMN: a retrospective, single-center observational study. BMC Surg 23, 3 (2023). https://doi.org/10.1186/s12893-023-01902-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-023-01902-1