Abstract
Background
Self-repair of lower limb wounds has always been one of the research hotspots. Flaps and skin graft are the preferred treatment for lower extremity wound reconstruction. However, these treatments have many disadvantages, such as secondary damage, poor healing quality. In recent years, the use of acellular dermal matrix has emerged as an alternative treatment option for extremity ulcers.
Methods
This study aimed to explore whether acellular dermal matrix can be used as a single treatment to promote wound healing. 7 patients with lower extremities cutaneous deficiency exposing bone or tendon, were covered by Pelnac, which was an acellular dermal matrix product approved by China Food and Drug Administration. All the wound was treated by Pelnac without flaps and skin graft. The external dressing was changed every 10 days.
Results
After a maximum of 20 weeks, all the wounds were completely healed. During the 12 months follow-up period none of the patients developed skin wear on the treatment area. All patients maintained their postoperative ambulatory ability. All patients were satisfied with the appearance and feeling after wound healing.
Conclusion
These findings may mean acellular dermal matrix is a novel method offering opportunity for treatment of lower extremities cutaneous deficiency exposing bone or tendon. It also has the potential to close wounds of all uninfected, non-ischemic, full-thickness cutaneous deficiency.
Similar content being viewed by others
Introduction
The skin is the largest organ covering the human body. Continuous loss of normal anatomic structures and function of the skin results in a wound [1, 2]. Based on their wound healing time frames, wounds are classified as acute or chronic. In everyday pathology, wounds remain a challenging clinical problem, with early and late complications representing a frequent cause of morbidity and mortality [3, 4]. Acute wounds constitute a common health problem, with an estimated 11.8 million wounds treated in emergency departments in the USA annually [5, 6]. Wounds are typically characterized based on wound depth and the area of skin affected [7]. According to the degree of skin injury, wounds can be divided into partial-thickness wounds and full-thickness wounds [8]. There are numerous therapeutic methods used to treat different types of wounds, such as negative pressure wound therapy, bioengineered tissue alternatives, pedicle flaps, and free tissue transfer. Based on the “reconstruction pyramid”, which is a guide for wound healing, treatments are arranged hierarchically, from minimally invasive procedures for small and superficial wounds to highly complex procedures for large, deep, and complex soft tissue defects [9]. For instance, due to a lack of redundant or pliable surrounding soft tissue, wounds in the foot or ankle often need to be closed with various kinds of flaps. However, surgical flap interventions have associated disadvantages that include secondary damage, poor tissue appearance, and high surgical requirements. For this reason, tissue engineered dressings have better application prospects than flap surgery. Acellular dermal matrix (ADM) is a promising tool for the treatment of soft tissue defects that can maintain an enhanced quality of wound repair [10,11,12]. In recent years, acellular dermal matrix (ADM) has been used in the treatment of deep tissue defects in combination with split-thickness skin grafts and negative pressure wound therapy [13, 14]. However, ADM also has the disadvantage that it cannot independently achieve physiological epithelial tissue regeneration and repair [15]. Here, we found that a porcine acellular dermal matrix can promote complete wound healing.
Materials and methods
All patients provided informed consent to participate in the study and for the use of their images. Inclusion criteria: full-thickness skin defect below the knee joint. Exclusion criteria: Patients with diabetes mellitus, lower extremity vascular disease or osteomyelitis. We used Pelnac (Gunze Corp., Kyoto, Japan) (an artificial dermal product approved by the China Food and Drug Administration) for wound treatment. The Pelnac double layer is composed of an atelocollagen sponge layer and a silicone layer.
Seven patients with lower extremity cutaneous deficiency with exposed bone or tendon were treated in our department from December 2014 to January 2017. This prospective study included a 12-month follow-up, with an overall study period of 3 years. Patient age ranged between 8 and 55 years (mean age 40.6 years). None of the patients had concomitant flap or fascia flap surgery, and none presented with vascular disease or diabetes. Postoperative sensory status was evaluated through two-point discrimination measurements and assessments of total active motion.
All surgical procedures were performed under epidural anesthesia with the patient in a supine position. A tourniquet was placed around the lower limb to limit intraoperative bleeding. After debridement and hemostasis, the wound was rinsed three times with H2O2 and 0.1% povidone iodine. Afterwards, the wound was covered with Pelnac according to the manufacturer’s protocol. Subsequently, the Pelnac was trimmed to precisely contour the wound and then immersed in saline for 15 s before application. The Pelnac was sutured to the defect using 5/0 Prolene sutures. To facilitate wound exudation, small drainage holes were made in the silicone film. After the operation, gauze and bandages were applied to cover the surface of the Pelnac. The dressings were changed every 2–5 days depending on the exudation, and outpatient or online visits were performed every 2–3 weeks. The silicone film was only detached from the wound after complete healing.
Statistical analysis
Statistical analysis consisted of the comparison between the affected side and healthy side in terms of two-point discrimination measurements and total active motion, performed using the Wilcoxon signed-rank test. The level of statistical significance was set at p < 0.05.
Results
In this study, 7 patients (age range 6–62 years) were treated with Pelnac for lower extremity wounds with bone or tendon exposure (wound size range 5–49.5 cm2). All wounds resulted from tissue removal and debridement, and defects were filled by suturing the Pelnac in place. Final wound healing occurred within 13 weeks on average (range 7–20 weeks). One or two linear scars [16] were left in the original wound area, depending on wound shape. The width of the scars varied from 1 to 15 mm depending on the initial size of the wounds.
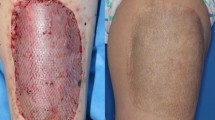
After 12 months of follow-up, skin sensation and joint movement were evaluated in all patients. All patients achieved a return to normal sensation, although slight variations in sensation between the healthy side and the affected side were noted in a few instances. At the one-year follow-up, the healed wound was free of significant pain, and the range of joint motion averaged 90% of that of the healthy side (Table 1). None of the patients showed signs of skin damage or trouble walking. All patients returned to normal daily life. Specific cases can be seen in Figs. 1, 2, 3.
Discussion
ADM has long been used as soft tissue replacement therapy in the field of wound healing and tissue repair and reconstruction [17]. Commonly, the use of ADM to repair wounds has been combined with skin grafting or negative-pressure wound therapy (NPWT) [14, 18]. A handful of studies have reported regeneration of full-thickness skin defects using ADM, without skin graft or flap surgery [19,20,21]. As a clinical challenge, chronic vascular ulcers need to be treated by various surgical methods [22]. However, ADM also has advantages in the treatment of chronic vascular ulcers and can improve the healing rate and wound closure rate [23]. Pelanc, a double-layer structure of ADM, is generally used as a pretreatment for skin grafting, can improve the survival rate of skin grafting and accelerate wound healing [24]. We treated a case of hand trauma with a skin defect on the palm. However, the patient did not take the doctor's advice to undergo a second skin grafting surgery. At his next outpatient appointment approximately 50 days later, we found that the wound had healed. Based on this case, it is possible that ADM can not only serve as an anatomical dressing but also has the ability to stimulate wound repair. The possible mechanism is that ADM, which is similar to the dermal reticular structure, reduces the accumulation of collagen, induces angiogenesis and remodels the structure and function of the dermis [25].
In this article, we show that ADM can replace skin grafts and flaps to promote the regeneration of full-thickness skin defects. Although contraction of the wound cannot be completely avoided due to the poor extensibility of the skin at these injured sites, it is still possible that the wound can healed by regeneration. Furthermore, we found that PELNAC has certain anti-infective ability. Although the wound initially appears to resemble skin necrosis or pus formation after being covered with PELNAC for a period of time, it then starts to become drier, and the Pelnac eventually promotes regeneration. This is consistent with the results of our current animal experiments that showed that the infection rate of the wound was reduced after the use of ADM.
Compared with skin grafts and flaps, ADM presents some distinct advantages. First, patients treated with ADM are unconstrained by donor supply or damage. None of the patients needed skin or flaps harvested from other parts of the body. Second, the quality of the wounds treated with ADM was better than that of the skin or flaps in the surgical arm of the study. The comparison between affected and healthy limbs indicated comparable passive (or active) motion of the lower limb joints. In addition, the regenerated skin healed and demonstrated a satisfactory appearance and feel. After 1 year, according to the two-point discrimination measurements, the recovery with Pelnac was better than that with skin grafting [26].
However, there are shortcomings in the application of ADM to treat trauma, such as the long treatment time, which often takes several months and is a challenge for patients’ patience and trauma care. Specimens were not sampled from healed wounds, thereby limiting further pathological analysis. However, previous work conducted in our laboratory found concomitant hair regeneration for a few wounds treated with ADM [20]. These rat studies showed the infiltration of stem cells following treatment, but further work is required to determine a detailed mechanism of action.
Conclusion
In conclusion, for select patients, 1-stage Pelnac reconstruction can be considered a novel method for inducing regrowth of the epidermis, thus warranting further research.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–93. https://doi.org/10.1001/archderm.1994.01690040093015Medline.
Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38(2):A1–140. https://doi.org/10.1067/msg.2001.111167Medline.
Natarajan S, Williamson D, Stiltz AJ, Harding K. Advances in wound care and healing technology. Am J Clin Dermatol. 2000;1(5):269–75. https://doi.org/10.2165/00128071-200001050-00002Medline.
Alonso JE, Lee J, Burgess AR, Browner BD. The management of complex orthopedic injuries. Surg Clin North Am. 1996;76(4):879–903. https://doi.org/10.1016/S0039-6109(05)70486-2Medline.
Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data. 2007;386:1–32.
Singer AJ, Dagum AB. Current management of acute cutaneous wounds. N Engl J Med. 2008;359(10):1037–46. https://doi.org/10.1056/NEJMra0707253Medline.
Krasner D, Kennedy KL, Rolstad BS, Roma AW. The ABCs of wound care dressings. Ostomy/wound Manag. 1993;39(8):66.
Chhabra S, Chhabra N, Kaur A, Gupta N. Wound healing concepts in clinical practice of OMFS. J Maxillofac Oral Surg. 2017;16(4):403–23. https://doi.org/10.1007/s12663-016-0880-zMedline.
Capobianco CM, Stapleton JJ, Zgonis T. Soft tissue reconstruction pyramid in the diabetic foot. Foot Ankle Spec. 2010;3(5):241–8. https://doi.org/10.1177/1938640010375113Medline.
Srivastava A, DeSagun EZ, Jennings LJ, Sethi S, Phuangsab A, Hanumadass M, et al. Use of porcine acellular dermal matrix as a dermal substitute in rats. Ann Surg. 2001;233(3):400–8. https://doi.org/10.1097/00000658-200103000-00015Medline.
Chern PL, Baum CL, Arpey CJ. Biologic dressings: current applications and limitations in dermatologic surgery. Dermatol Surg. 2009;35(6):891–906.
Tang B, Zhu B, Liang YY, Bi LK, Chen B, Hu ZC, et al. Early escharectomy and concurrent composite skin grafting over human acellular dermal matrix scaffold for covering deep facial burns. Plast Reconstr Surg. 2011;127(4):1533–8. https://doi.org/10.1097/PRS.0b013e31820a63e8Medline.
Son JH, Bafus B, Khandelwal A, Chepla KJ. Reconstruction of a circumferential upper extremity soft tissue defect with a dermal regeneration template and skin grafting. Tech Hand Up Extrem Surg. 2018;22(1):31–3. https://doi.org/10.1097/BTH.0000000000000185Medline.
Lee YJ, Park MC, Park DH, Hahn HM, Kim SM, Lee IJ. Effectiveness of acellular dermal matrix on autologous split-thickness skin graft in treatment of deep tissue defect: esthetic subjective and objective evaluation. Aesthetic Plast Surg. 2017;41(5):1049–57. https://doi.org/10.1007/s00266-017-0891-2Medline.
Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4(14):413–37. https://doi.org/10.1098/rsif.2006.0179Medline.
van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by the patient and observer scar assessment scale. Plast Reconstr Surg. 2005;116(2):514–22. https://doi.org/10.1097/01.prs.0000172982.43599.d6.17.
Jansen LA, De Caigny P, Guay NA, Lineaweaver WC, Shokrollahi K. The evidence base for the acellular dermal matrix alloderm a systematic review. Ann Plast Surg. 2013;70(5):587–94. https://doi.org/10.1097/SAP.0b013e31827a2d23Medline.
Pontell ME, Saad N, Winters BS, Daniel JN, Saad A. Reverse sural adipofascial flaps with acellular dermal matrix and negative-pressure wound therapy. Adv Skin Wound Care. 2018;31(1):612–7. https://doi.org/10.1097/01.ASW.0000527290.81581.afMedline.
Girod DA, Sykes K, Jorgensen J, Tawfik O, Tsue T. Acellular dermis compared to skin grafts in oral cavity reconstruction. Laryngoscope. 2009;119(11):2141–9. https://doi.org/10.1002/lary.20548Medline.
Lou X, Xue H, Li G, Wang K, Zhou P, Li B, et al. One-stage pelnac reconstruction in full-thickness skin defects with bone or tendon exposure. Plast Reconstr Surg Glob Open. 2018;6(3): e1709. https://doi.org/10.1097/GOX.0000000000001709Medline.
De Angelis B, Gentile P, Tati E, Bottini DJ, Bocchini I, Orlandi F, et al. One-stage reconstruction of scalp after full-thickness oncologic defects using a dermal regeneration template (Integra). BioMed Res Int. 2015;2015:1–11. https://doi.org/10.1155/2015/698385Medline.
Cigna E, Pierazzi DM, Sereni S, Marcasciano M, Losco L, Bolletta A. Lymphatico-venous anastomosis in chronic ulcer with venous insufficiency: a case report. Microsurgery. 2021;41(6):574–8. https://doi.org/10.1002/micr.30753.Medline.
Cazzell S. A randomized controlled trial comparing a human acellular dermal matrix versus conventional care for the treatment of venous leg ulcers. Wounds. 2019;31(3):68–74.
Lisa A, Galtelli L, Vinci V, Veronesi A, Klinger M. Adoption of a newly introduced dermal matrix: preliminary experience and future directions. BioMed Res Int. 2020;2020:1–5. https://doi.org/10.1155/2020/3261318.
Tork S, Jefferson RC, Janis JE. Acellular dermal matrices: applications in plastic surgery. Semin Plast Surg. 2019. https://doi.org/10.1055/s-0039-1693019.
Mehra R, Langer V, Manral S. A prospective study on return of protective tactile sensations in split-thickness skin grafts. Inadian J Surg. 2020;82(3):350–4. https://doi.org/10.1007/s12262-019-01965-6.
Acknowledgements
Not applicable.
Funding
We gratefully acknowledge funding from the National Natural Science Foundation of China (NSFC, 81801922), Chronic Wound & Diabetes Medical Clinical Research Center Foundation of Hubei provincial China (2018BCC340) and Hubei Province key research and development program (2020BCB029, 2020BCB031).
Author information
Authors and Affiliations
Contributions
GL and QS wrote the main manuscript text. PZ and HL prepared original draft. JH review and editing. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Hospital Ethics Committee of the Huazhong University of Science and Technology of Tongji Medical College. All of the research processes were followed the principles outlines in the Declaration to Helsinki. All patients provided informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
No conflict of interest exits in the submission of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, G., Shen, Q., Zhou, P. et al. Acellular dermal matrix for one-stage treatment of lower extremity full-thickness skin defect: a case series. BMC Surg 23, 17 (2023). https://doi.org/10.1186/s12893-022-01871-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01871-x