Abstract
Background
Adjunct hemostats can be of use in certain surgical settings. We compared the effectiveness of two hemostats, Hemopatch® and Surgicel® Original in controlling bleeding from liver lesions in an experimental model.
Methods
Control of grades 1 (mild) and 2 (moderate) bleeding (according to the Validated Intraoperative Bleeding [VIBe] SCALE) was assessed for 10 min after Hemopatch® (n = 198) or Surgicel® Original (n = 199) application on 397 liver surface lesions. The primary endpoint was hemostatic success (reaching VIBe SCALE grade 0 at 10 min). The secondary endpoint was time to hemostasis (time to reach and maintain grade 0). A generalized linear mixed model and an accelerated failure time model were used to assess the primary and secondary endpoints, respectively.
Results
The overall hemostatic success rate of Hemopatch® was statistically significantly superior to that of Surgicel® Original (83.8% versus 73.4%; p = 0.0036; odds ratio [OR] 2.38, 95% confidence interval [CI] 1.33–4.27) and time to hemostasis was reduced by 15.9% (p = 0.0032; 95% CI 0.749–0.944). Grade 2 bleeds treated with Hemopatch® had statistically significantly higher hemostatic success (71.7% versus 48.5%; p = 0.0007; OR 2.97, 95% CI 1.58–5.58) and shorter time to hemostasis (49.6% reduction, p = 3.6 × 10–8); differences for grade 1 bleeds (hemostatic success rate or time to hemostasis) were not statistically significant.
Conclusions
Hemopatch® provided better control of VIBe SCALE bleeding compared to Surgicel® Original for Grade 2 bleeds in this porcine model, highlighting the importance of choosing a suitable hemostat to optimize control of bleeding during surgery.
Similar content being viewed by others
Background
Management of bleeding is a critical outcome factor during surgical procedures [1]. Effective management of intraoperative and postoperative bleeding reduces the risk of complications, morbidity and mortality and treatment costs, especially those associated with blood and blood product transfusion [2]. The percentage of patients with bleeding-related complications varies by specialty, but overall, it can be as high as 28.5% in general and solid organ procedures and 47.4% in cardiac surgery [3]. Increasing numbers of cancer operations in more complex and advanced stages [4] and patients on anticoagulation and antiplatelet treatment may lead to a higher risk of intra- and postoperative bleeding [5, 6]. Similarly, bleeding management is also crucial when treating patients with visceral trauma and non-traumatic emergencies [7, 8]. Appropriately selected adjunct topical hemostats can help reduce the perioperative risk of bleeding [9] and therefore, might be useful, especially for the treatment of organ lacerations [10].
The purpose of this study was to evaluate the hemostatic effectiveness of two widely used hemostats, an N-hydroxysuccinimide functionalized polyethylene glycol-coated collagen patch (Hemopatch®, Baxter Healthcare Corporation, Deerfield, IL, USA) and an absorbable knitted oxidized regenerated cellulose fabric (Surgicel® Original, Ethicon Inc, Bridgewater NJ, USA), in Validated Intraoperative Bleeding (VIBe) SCALE [11] mild and moderate bleeding when applied to surgically induced liver lesions in a heparinized porcine model. Both hemostats are indicated for use in hepatobiliary procedures and are frequently used in this setting [12,13,14,15,16,17,18]. To our knowledge, there are currently no reports available that compare these adjunct hemostats using a surgeon validated bleeding scale to objectively determine effectiveness in specific bleeding severities.
Methods
Study design
Two hemostats were investigated: Hemopatch® and Surgicel® Original. Since adjunct hemostats have recently gained market approval for use in mild and moderate bleeding [19, 20], the treatment of VIBe SCALE grades 1 and 2 bleeding [14] was investigated.
Treatments were assigned to each target application site by a block randomization scheme created using the “blockrand” 17 package of the R Software (R Core Team, Austria, Vienna) [21, 22].
Animals
Thirty-five healthy castrated male naïve Yorkshire cross swine (mean weight 55 ± 6.4 kg) were used. Animals were group housed in stainless steel pens on raised flooring and single housed for fasting, on a 12 h light/dark cycle. Temperature and humidity were kept within 18–25 °C and 30–70% ranges, respectively. Animals were fed a certified pig diet twice daily and were fasted overnight prior to surgery. Tap water was available ad libitum. All animals were observed daily for general health and signs of disease.
Surgical procedure
Animals were anesthetized by veterinary anesthesiologists with intramuscular tiletamine/zolazepam (4.05–5.03/2.02–2.51 mg/kg), intramuscular xylazine (1.5–3.5 mg/kg), intravenous propofol (2–8 mg/kg) and isoflurane inhalation. Analgesia consisted of intramuscular buprenorphine (0.04–0.05 mg/kg) and lidocaine (0.5–2.0 mg/kg), administered subcutaneously along the incision.
Animals were placed in dorsal recumbency. A carotid artery catheter was placed for invasive blood pressure monitoring purposes, and a jugular vein line ensured fluid and supportive therapy administration as well as intraoperative blood sampling. A midline laparotomy was performed, and the liver was positioned for optimal exposure and maximal testing surface availability. Heparin was administered intravenously prior to lesion creation and throughout the hemostasis evaluation period as needed to maintain an activated clotting time of approximately twice that of baseline and a maximum of 600 s, thus creating a standardized impaired coagulation status across treatment groups [23]. Clotting time was measured at least every 25 min.
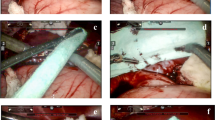
An electrocautery scratch pad was used to create lesions on the liver surface resulting in mild bleeding, which was defined as oozing or intermittent capillary-like bleeding at a rate of > 1.0–5.0 mL/min (VIBe SCALE grade 1; Fig. 1). Square lesions (approximately 1 × 1 cm, up to 4 mm deep) were created using sharp dissection. Liver tissue was removed using sharp and blunt dissection, maintaining the targeted depth within the squares to create moderate bleeding, which was defined as continuous flow bleeding at a rate of > 5.0–10.0 mL/min (VIBe SCALE grade 2; Fig. 1). Lesion pairs were created and assessed for 10 min after treatment prior to creating a new set of lesions. According to available anatomical space and animal physiological responses throughout the procedure, the median number of lesions per animal was 12. Only confirmed grades 1 or 2 bleeding lesions were included in the final analysis. All animals were euthanized after hemostasis evaluation.
VIBe SCALE. *Systemic resuscitation was required (e.g., volume expanders, vasopressors, blood products, etc.). Adapted with permission of Elsevier Publishers at no cost to reproduce the VIBe SCALE table (in line with the BMC Surgery guidelines) from Surgery 161 (3): Lewis et al. [11]. Copyright Elsevier 2017
Assessment of bleeding
Bleeding was assessed by the study surgeon using the VIBe SCALE [11]. This scale classifies bleeding into 5 grades (ranging from 0: no bleed, to 4: life threatening) as shown in Fig. 1.
Pretreatment blood loss rate was determined quantitatively by measuring the amount of blood absorbed by a pre-weighed gauze in 10 s and calculating the blood loss rate per minute (1–5 mL/min for grade 1 and 5–10 mL/min for grade 2). Additionally, blood loss rate was measured for each treatment 10 min after Hemopatch® or Surgicel® Original application to determine whether hemostasis was achieved (VIBe SCALE grade 0, ≤ 1 mL/min). Potential bias was controlled by blinding the study surgeon to the treatment arm until the lesion was created and the VIBe SCALE grade was determined. Additionally, blood loss rates prior to treatment and at the primary endpoint assessment were quantified to confirm VIBe SCALE grades. Each treatment arm evaluated the effectiveness of Hemopatch® and Surgicel® Original for treating grades 1 and 2 bleeding combined, as well as separately.
Application methods
Both Hemopatch® and Surgicel® Original were applied in single layers to the bleeding sites, overlapping the bleeding surface by at least 1 cm in accordance with their respective instructions for use [24, 25]. If hemostasis was not achieved at the pre-determined time points, additional pressure with gauze for 30-s intervals was allowed but no additional Hemopatch® was applied during the 10-min hemostasis evaluation period. For VIBe SCALE grade 2 bleeding sites, if hemostasis was not achieved at one of the pre-determined time points (2, 3, 5, and 7 min after application) a second layer of Surgicel® Original could be applied (for a total of two layers), followed by additional pressure/approximation using dry gauze for 30-s intervals, or until the next pre-determined evaluation time point.
Study endpoints
The primary endpoint was hemostatic success, defined as achieving VIBe SCALE grade 0 (Fig. 1) 10 min after application. An observation period of 10 min was selected to include the anticipated time to hemostasis for the adjunct hemostats as well as a clinically acceptable intraoperative period to monitor and assure maintenance of hemostasis. The secondary endpoint was time to hemostasis, defined as the time post-treatment that the treated lesion reached and maintained grade 0. Lesions were evaluated at 2, 3, 5, 7 and 10 min after the start of treatment.
Statistical analysis
A sample size of 197 lesions per treatment (a total of 394 lesions) was calculated to provide 80% power to detect a difference of 0.10 (calculated using PASS 2019, NCSS, East Kaysville, UT, USA). Logistic generalized linear mixed models were used to evaluate hemostatic success for overall (VIBe SCALE grades 1 and 2 bleeding combined), grade 1 bleeding, and grade 2 bleeding. The model included hemostatic success as the dependent variable, treatment as a fixed variable, pretreatment (baseline) bleeding grade as a fixed covariate (for overall bleeding only), and animal as a random effect. For analysis of time to hemostasis, an accelerated failure time model was used to model the difference in time to hemostasis between Hemopatch® and Surgicel® Original. The overall model included the fixed variables treatment and baseline bleeding grade (grade 1 or grade 2 bleeding), and animal as a random effect. Kaplan–Meier estimates of the median times to hemostasis between the two products were compared by the log-rank test. R software version 3.6.1 [22] was used to perform all analyses. P-values less than or equal to 0.05 were considered statistically significant.
Results
Hemostatic effectiveness was evaluated on 198 and 199 lesions treated with Hemopatch® and Surgicel® Original, respectively. Pretreatment blood loss rates ranged from 1.02–4.74 to 5.04–9.96 mL/min for VIBe SCALE grade 1 and grade 2, respectively.
Hemostatic success
The hemostatic success of Hemopatch® at 10 min (primary endpoint) was statistically significantly superior to that of Surgicel® Original for VIBe SCALE grades 1 and 2 combined (166/198 [83.8%] versus 146/199 [73.4%]; p = 0.0036; odds ratio [OR] 2.38, 95% confidence interval [CI] 1.33–4.27). In addition, Hemopatch® demonstrated greater hemostatic effectiveness at 2, 3, 5, and 7 min (Table 1).
For treatment of grade 1 bleeding, no statistically significant difference was found between the hemostatic success rates at 10 min for Hemopatch® and Surgicel® Original (95/99 [96.0%] versus 98/100 [98.0%]; p = 0.3396; OR 0.37, 95% CI 0.05–2.83). In contrast, for grade 2 bleeding the hemostatic success rate at 10 min for Hemopatch® was statistically significantly higher than that of Surgicel® Original (71/99 [71.7%] versus 48/99 [48.5%]; p = 0.0007; OR 2.97, 95% CI 1.58–5.58).
For VIBe SCALE bleeding grade 2, only 6.1% (6/99) lesions treated with a single layer of Surgicel® Original achieved hemostasis. For the remaining lesions an additional layer of Surgicel® Original was applied, of which 54.8% (51/93) still did not achieve hemostasis within the 10-min observation period.
Time to hemostasis
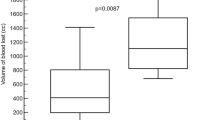
For treatment of VIBe SCALE grades 1 and 2 bleeding combined, the time to hemostasis when using Hemopatch® was 15.9% shorter than that of Surgicel® Original (p = 0.0032; fold-change 0.841, 95% CI 0.749–0.944). The median time to hemostasis for combined grades 1 and 2 bleeding was 2 min for both the Hemopatch® and Surgicel® Original treatments (Table 2); however, the 95% CI for the median was narrower for Hemopatch® [2–2 min] than that for Surgicel® Original [2–5 min]. The probability of continued bleeding was statistically significantly lower for Hemopatch® than for Surgicel® Original at every time point other than 10 min (as shown by the non-overlapping 95% CIs; Fig. 2A).
For treatments of VIBe SCALE grades 1 and 2 bleeding evaluated separately, the time to hemostasis when using Hemopatch® was not statistically significantly different than that of Surgicel® Original for grade 1 bleeding (p = 0.51; fold-change 1.023, 95% CI 0.956–1.096); however, for grade 2 bleeding the time to hemostasis when using Hemopatch® was approximately half that of Surgicel® Original (p = 3.6 × 10–8; fold-change 0.504, 95% CI 0.395–0.644). The median time to hemostasis for grade 1 bleeding for both treatments was 2 min (95% CI 2–2 min) (Table 2). For grade 1 bleeding, there was no statistically significant difference in the probability of continued bleeding at all time points between the two hemostats (Fig. 2B). The median time to hemostasis for grade 2 bleeding treated with Hemopatch® was 3 min (95% CI 2–7 min). The median time to hemostasis and 95% CI for grade 2 bleeding treated with Surgicel® Original could not be computed because more than 50% of the grade 2 lesions did not achieve hemostasis (Table 2). The probability of continued bleeding of grade 2 lesions was statistically significantly lower for lesions treated with Hemopatch® than for those treated with Surgicel® Original at all time points (as shown by the non-overlapping 95% CIs; Fig. 2C).
Discussion
This study showed that overall, using Hemopatch® to treat VIBe SCALE-defined mild and moderate bleeding in this heparinized porcine hepatic bleeding model increased (statistically significantly for grade 2 bleeding) the hemostatic success rate and resulted in statistically significantly shorter time to hemostasis compared to Surgicel® Original.
Given the increasing number of patients receiving anticoagulant prescriptions for long-term management of cardiac, cerebrovascular or peripheral vascular conditions [26, 27], surgeons frequently encounter patients taking these medications in both elective and emergency surgery settings, possibly leading to increased blood loss, longer operative times, greater use of surgical consumables, and higher costs [2]. Heparinization was therefore used in this study to mimic these clinical scenarios as well as intraoperative anticoagulation of patients. Active hemostats, such as Hemopatch®, are effective in patients with impaired coagulation, such as those receiving anticoagulation or antiplatelet therapies [28], whereas the efficacy of passive hemostats, such as Surgicel® Original is reduced in these patients [9, 28].
While a network meta-analysis study did not show that general use of local hemostatic agents reduces the rate of clinically relevant bleeding in thyroid surgery [29], a retrospective analysis of cardiac, vascular, noncardiac thoracic, solid organ, general reproductive organ, knee/hip replacement, spinal, and neurosurgical data from the Premier US Perspective Hospital Database reported fewer bleeding-related complications and shorter hospital/intensive care unit stays across all specialties when surgical bleeding was treated immediately with active hemostats, compared with treatment using a combination of passive and active hemostats [30], in which the passive hemostat is typically employed first. Combined with the results of a previous study of severe aortic bleeding [31], our results suggest that Hemopatch® is effective at achieving hemostasis across a broad range of bleeding severities. Taken together, this suggests that an active hemostat treatment strategy may be preferable across a range of surgical settings and where passive hemostats are less advisable based on their mechanism of action [32, 33].
One of the possible explanations we did not find any statistically significant difference in Grade 1 bleeding might be that manual pressure alone is sufficient in some instances of mild bleeding.
Given the increasing importance of bleeding management during surgery, discussed above, it is necessary to obtain objective evidence of a hemostat’s effectiveness in different settings and bleeding severities to enable surgeons to make appropriate treatment decisions. Semiquantitative scoring, quantification of the blood loss rate, or visual description of bleeding are frequently used in pre-clinical [32] and clinical settings [14, 33, 34], but these methods lack standardization across investigators and studies. Utilizing the VIBe SCALE helps to overcome these limitations as it is surgeon validated and can be easily interpreted across a broad range of surgical specialties [11].
The main limitation of this animal study is the proof of the clinical relevance of the results, although the porcine hepatic bleeding model is an accepted method for assessing performance of adjunct hemostats that have since been approved for use in patients [35, 36]. Hemopatch® is a thin, pliable collagen patch with NHS-PEG coating to facilitate sealing and hemostasis, which may provide an advantage for bleeding on the liver surface. Physical properties and practicality should be considered when selecting an adjunct topical hemostat for different clinical scenarios.
Conclusions
This study showed that treating VIBe SCALE-defined mild and moderate bleeding with Hemopatch® increased the hemostatic success rate and decreased bleeding times compared to Surgicel® Original. In the context of increasing anticoagulant use and the evolving complexity of surgical procedures, the results of this study highlight the importance of choosing a suitable hemostat, whenever applicable, to optimize hemostasis during surgery.
Availability of data and materials
The data that support the findings of this study are available from Baxter Healthcare Corporation but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Baxter Healthcare Corporation.
Abbreviations
- CI:
-
Confidence interval
- OR:
-
Odds ratio
- VIBe SCALE:
-
Validated Intraoperative Bleeding SCALE
References
Marietta M, Facchini L, Pedrazzi P, Busani S, Torelli G. Pathophysiology of bleeding in surgery. Transplant Proc. 2006;38(3):812–4.
Ramirez MG, Castillo GF, Ramirez MA. The economic burden of bleeds and transfusions in selected surgeries: a retrospective multi center analysis from the US perspective. Am J Biomed Sci Res. 2019;2(5):211–6.
Stokes ME, Ye X, Shah M, Mercaldi K, Reynolds MW, Rupnow MF, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
Markham MJ, Wachter K, Agarwal N, Bertagnolli MM, Chang SM, Dale W, et al. Clinical cancer advances 2020: annual report on progress against cancer from the American society of clinical oncology. J Clin Oncol. 2020;38(10):1081.
Lock JF, Ungeheuer L, Borst P, Swol J, Löb S, Brede EM, et al. Markedly increased risk of postoperative bleeding complications during perioperative bridging anticoagulation in general and visceral surgery. Perioper Med (Lond). 2020;9(1):39.
Staerkle RF, Hoffmann H, Köckerling F, Adolf D, Bittner R, Kirchhoff P. Does coagulopathy, anticoagulant or antithrombotic therapy matter in incisional hernia repair? Data from the Herniamed registry. Surg Endosc. 2018;32(9):3881–9.
Hooper N, Armstrong TJ. Hemorrhagic shock. StatPearls. Treasure Island (FL): StatPearls Publishing, Copyright © 2021, StatPearls Publishing LLC.; 2021.
Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23(1):98.
Chiara O, Cimbanassi S, Bellanova G, Chiarugi M, Mingoli A, Olivero G, et al. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018;18(1):68.
King DR, Cohn SM, Proctor KG. Modified rapid deployment hemostat bandage terminates bleeding in coagulopathic patients with severe visceral injuries. J Trauma. 2004;57(4):756–9.
Lewis KM, Li Q, Jones DS, Corrales JD, Du H, Spiess PE, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017;161(3):771–81.
Di Cesare T, Scarinci A, Cavaniglia D, Brun S, Cilurso F, Cosenza G, et al. Efficacy of Hemopatch® in reducing postoperative bleeding after laparoscopic cholecystectomy: prospective and multicenter study. Int Clin Med. 2018;2:1–3.
Fingerhut A, Uranues S, Ettorre GM, Felli E, Colasanti M, Scerrino G, et al. European initial hands-on experience with HEMOPATCH, a novel sealing hemostatic patch: application in general, gastrointestinal, biliopancreatic, cardiac, and urologic surgery. Surg Technol Int. 2014;25:29–35.
Fischer CP, Bochicchio G, Shen J, Patel B, Batiller J, Hart JC. A prospective, randomized, controlled trial of the efficacy and safety of fibrin pad as an adjunct to control soft tissue bleeding during abdominal, retroperitoneal, pelvic, and thoracic surgery. J Am Coll Surg. 2013;217(3):385–93.
Lewis KM, Ikeme S, Olubunmi T, Kuntze CE. Clinical effectiveness and versatility of a sealing hemostatic patch (HEMOPATCH) in multiple surgical specialties. Expert Rev Med Devices. 2018;15(5):367–76.
Lopez-Guerra D, Santos-Naharro J, Rojas-Holguin A, Jaen-Torrejimeno I, Prada-Villaverde A, Blanco-Fernandez G. Postoperative bleeding and biliary leak after liver resection: a cohort study between two different fibrin sealant patches. Sci Rep. 2019;9(1):12001.
Masci E, Faillace G, Longoni M. Use of oxidized regenerated cellulose to achieve hemostasis during laparoscopic cholecystectomy: a retrospective cohort analysis. BMC Res Notes. 2018;11(1):239.
Olthof PB, Rassam F, van Gulik TM. The use of a NHS-PEG coated, collagen-based sealant in a patient undergoing associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Int J Surg Case Rep. 2018;47:7–10.
HEMOBLAST (TM) bellows hemostatic agent prescribing information. https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170012C.pdf. Accessed 18 Aug 2021.
VISTASEAL (TM) hemostatic agent prescribing information. https://www.fda.gov/media/108694/download. Accessed 18 Aug 2021.
Snow G. blockrand: randomization for block random clinical trials. R package version 1.5. https://CRAN.R-project.org/package=blockrand. 2020.
Team RC. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. 2019.
Mabry CD, Thompson BW, Read RC. Activated clotting time (ACT) monitoring of intraoperative heparinization in peripheral vascular surgery. Am J Surg. 1979;138(6):894–900.
Surgicel original product information. www.healthproductsforyou.com/ProdImages/CommonFile/Ethicon-Surgicel-Absorbable-Hemostat_User-Manual.pdf. Accessed 21 June 2022.
Hemopatch® instructions for use. https://cnchirurgie.ro/wp-content/uploads/2020/10/Hemopatch.pdf. Accessed 21 June 2022.
Colacci M, Tseng EK, Sacks CA, Fralick M. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35(8):2505–7.
Orlowski A, Gale CP, Ashton R, Petrungaro B, Slater R, Nadarajah R, et al. Clinical and budget impacts of changes in oral anticoagulation prescribing for atrial fibrillation. Heart. 2021;107(1):47–53.
Samudrala S. Topical hemostatic agents in surgery: a surgeon’s perspective. AORN J. 2008;88(3):S2-11.
Polychronidis G, Hüttner FJ, Contin P, Goossen K, Uhlmann L, Heidmann M, et al. Network meta-analysis of topical haemostatic agents in thyroid surgery. Br J Surg. 2018;105(12):1573–82.
Iannitti DA, Kim C, Ito D, Epstein J. Impact of an active hemostatic product treatment approach on bleeding-related complications and hospital costs among inpatient surgeries in the United States. J Med Econ. 2021;24(1):514–23.
Baumgartner B, Draxler W, Lewis KM. Treatment of severe aortic bleeding using hemopatch in swine on dual antiplatelet therapy. J Invest Surg. 2016;29(6):343–51.
Adams GL, Manson RJ, Hasselblad V, Shaw LK, Lawson JH. Acute in-vivo evaluation of bleeding with Gelfoam plus saline and Gelfoam plus human thrombin using a liver square lesion model in swine. J Thromb Thrombolysis. 2009;28(1):1–5.
Weltert L, D'Aleo S, Chirichilli I, Falco M, Turani F, Bellisario A, et al. Prospective randomized clinical trial of HEMOPATCH topical sealant in cardiac surgery. Surg Technol Int. 2016;XXIX:sti29/756.
Schuhmacher C, Pratschke J, Weiss S, Schneeberger S, Mihaljevic AL, Schirren R, et al. Safety and effectiveness of a synthetic hemostatic patch for intraoperative soft tissue bleeding. Med Devices (Auckl). 2015;8:167–74.
Lewis KM, Atlee HD, Mannone AJ, Dwyer J, Lin L, Goppelt A, et al. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Invest Surg. 2013;26(3):141–8.
MacDonald MH, Wang AY, Clymer JW, Hutchinson RW, Kocharian R. An in vivo comparison of the efficacy of hemostatic powders, using two porcine bleeding models. Med Devices (Auckl). 2017;10:273–9.
US Food and Drug Administration. FDA 21 CFR part 58 good laboratory practice regulations. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=58. Accessed July 2020.
Acknowledgements
Medical writing assistance was provided by Meridian HealthComms in accordance with good publication practice (GPP3), funded by Baxter Healthcare Corporation. Rebekka Molenaar provided assistance with statistical analyses.
Funding
This work was supported by funding from Baxter Healthcare Corporation.
Author information
Authors and Affiliations
Contributions
SU, AF, EL, DS, NR and BB contributed to the study design, data analysis, and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed at an Association for Assessment and Accreditation of Laboratory Animal Care-accredited institution following all applicable governmental regulations, including the U.S. Food and Drug Administration Code of Federal Regulations Title 21 Part 58 Good Laboratory Practice Regulations [37]. All experimental procedures were approved by the Animal Care and Use Committee of American Preclinical Services, USA. Methods used in this study are reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
S.U. and A.F. have received lecture honoraria from Baxter. E.L., D.S., N.R. and B.B. are employed by Baxter Healthcare Corporation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Uranues, S., Fingerhut, A., Levin, E. et al. Effectiveness of Hemopatch® versus Surgicel® Original to control mild and moderate liver bleeding. BMC Surg 22, 316 (2022). https://doi.org/10.1186/s12893-022-01747-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01747-0