Abstract
Background
The objective of this study was to investigate potential predictors of chemotherapy resistance in patients with advanced gastric cancer (GC) following radical gastrectomy.
Methods
Eligible stage II/III GC patients with adjuvant chemotherapy after radical gastrectomy were enrolled in this study. A receiver operating characteristic (ROC) curve analysis was performed to assess the predictive and optimal cut-off values of continuous variables for chemotherapy resistance. Potential risk factors for chemotherapy resistance were determined with binary univariate and multivariate analyses. Potential prognostic factors for overall survival (OS) were determined by COX regression analysis. The association between survival and AFR level was examined using the Kaplan–Meier curve analysis.
Results
A total of 160 patients were included in the data analysis, and 41 patients achieved chemotherapeutic resistance with an incidence of 25.6%. Pretreatment albumin/fibrinogen ratio (AFR) (cut-off value: 10.85, AUC: 0.713, P < 0.001) was a predictor for chemotherapeutic resistance by ROC curve analysis. Low AFR (< 10.85) was an independent risk factor of chemotherapeutic resistance as determined by the univariate and multivariate logistic regression analyses (OR: 2.55, 95%CI: 1.21–4.95, P = 0.005). Multivariate COX regression analyses indicated low AFR as a prognostic factor for 5-year OS (HR: 0.36, 95%CI: 0.15–0.73, P = 0.011). Low AFR was associated with poorer 5-year disease-free survival and overall survival.
Conclusions
This study indicated that a low level of pretreatment AFR could serve as an independent predictor of chemotherapy resistance and postoperative prognosis in GC patients following radical gastrectomy.
Similar content being viewed by others
Introduction
Globally, gastric cancer (GC) has the fourth incidence and third mortality rate [1]. As the third most common cancer in China, advanced GC is associated with a poor prognosis and relatively limited treatment options [1]. Although surgical resection remains the mainstay treatment for GC, the high postoperative recurrence incidence is a great threat to GC patients [2]. Thus, postoperative adjuvant chemotherapy is a quite important supplementary treatment [3]. In Asia, curative resection with D2 lymphadenectomy in combination with postoperative adjuvant chemotherapy remains the standard treatment option for stage II/III GC [4]. However, the clinical response to chemotherapy varies remarkably among advanced GC individuals, which may greatly influence the long-term prognosis [5]. Thus, there is an urgent need to explore potential predictors of chemotherapy response in advanced GC patients.
Albumin (Alb), an acute-phase reactant, is a protein that plays a critical role in regulating plasma oncotic pressure [6]. Fibrinogen (Fib), an acute-phase protein produced by the liver, is an important protein during the coagulation process, and it can aggregate in tumor sites [7]. Alb/Fib ratio (AFR), which combines Alb and Fib, has been widely used as a prognostic factor for several types of human malignancies [8]. This study aimed to investigate the potential ability of AFR in predicting the clinical response to chemotherapy and prognosis in GC patients.
Materials and methods
Patients
This was a retrospective, observational study which was approved by the Medical Institutional Ethics Committee of the researcher’s hospital (No. KY 201,408,701). This study was conducted in accordance with the Declaration of Helsinki. Eligible patients with advanced GC between 2014 and 2020 were consecutively included in this study. Inclusion criteria: (1) confirmed stage II or III GC with histological evidence; (2) scheduled to undergo postoperative chemotherapy following radical gastrectomy (D2, R0 resection); (3) Eastern Cooperative Oncology Group (ECOG) performance status [9] 0 or 1; (4) received at least 3 cycles of 5- fluorouracil (5-FU) based adjuvant chemotherapy; and (5) life expectancy ≥ 3 months. Exclusion criteria: (1) with stage I or IV GC or combined with other tumors; (2) with preoperative adjuvant treatment or postoperative radiotherapy; (3) with an autoimmune disease requiring systemic immunosuppressive treatment; (4) with the conditions affecting Alb and Fib expressions (e.g., hepatic dysfunction, hemopathy); (5) with contraindications to chemotherapy or could not tolerate chemotherapy due to the side effects or other reasons, (6) signet ring cell carcinoma or endocrine cell carcinoma, and (7) with incomplete data or loss to follow-up.
All enrolled patients received S-1 plus oxaliplatin (SOX) or capecitabine plus oxaliplatin (XELOX) chemotherapy regimens following the procedures by previous reports [10, 11]. Briefly, the SOX regimen involves 3-week cycles of 130 mg/m² intravenous oxaliplatin on day 1, oral S-1 based on the body surface area (BSA) (< 1.25 m², 80 mg daily; 1.25–1.5 m², 100 mg daily; ≥1.5 m², 120 mg daily) on days 1–14. The XELOX regimen involves 3-week cycles of 130 mg/m² intravenous oxaliplatin on day 1, oral capecitabine 1000 mg/ m² twice daily on days 1–14. The chemotherapy duration contains eight cycles (6 months).
Data collection
The following data were collected: (1) demographic data including age, sex distribution, body mass index (BMI), habits of smoking and drinking; (2) clinical baseline data including American Society of Anesthesiologists (ASA) grade, comorbidities of diabetes and hypertension, ECOG status; (3) treatment-related data including types of surgery, surgical approach, operation time, chemotherapy regimens, and cycles of chemotherapy; (4) tumor-related data including tumor location, tumor size, tumor differentiation, Lauren’s classification, clinical TNM stage, pathological TNM stage, and Her-2 status; (5) laboratory tests before the adjuvant treatment including hemoglobin (Hb), white blood cell (WBC), platelet, creatinine (Cr), urea, Alb, Fib, carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), CA19-9, and CA72-4. AFR was calculated by Alb divided by Fib. The diagnosis, clinical and histopathological stage of GC was confirmed according to the 8th edition of the Union for International Cancer Control/American Joint Committee (UICC/AJCC) classification [12].
Follow-up
The follow-up was performed in inpatient and outpatient every 3 months for the first 2 years, every 6 months thereafter. The follow-up assessments included physical examination, gastroscopy, laboratory tests, and radiologic assessment by computed tomography (CT). The primary endpoint was set as the proportion of patients who achieved chemotherapeutic resistance, which was defined as the progression of GC during chemotherapy or recurrence within 6 months of completed chemotherapy [13]. The secondary endpoint was set as 5-year disease-free survival (DFS, defined as the time from surgery to tumor relapse, death, or the 5-year due date), overall survival (OS, defined as the time from the diagnosis to death, or the 5-year due date).
Statistical analysis
All data were analyzed using GraphPad Prism 8.0 (GraphPad Inc., CA, USA) and SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Mean ± standard deviation (SD), or number with proportion (n, %) was used for data presentation. Chi-square or Fisher exact test was used for categorical data analysis, while Student t or Mann Whitney U test was used for measurement data analysis. A receiver operating characteristic (ROC) curve analysis was performed to assess the predictive and optimal cut-off values of continuous variables for clinical response to chemotherapy using the Youden index method. Potential risk factors for clinical response to chemotherapy were determined with binary univariate and multivariate analyses using the “Enter” method. Potential prognostic factors for OS were determined by COX regression analysis. The association between survival and AFR level was examined using the Kaplan–Meier curve analysis with the log-rank test. A p value of < 0.05 was considered statistically significant.
Results
There were 197 patients who met the inclusion criteria and were initially enrolled. Based on the exclusion criteria, 37 patients were then excluded (see the flow chart in Fig. 1) and a total of 160 patients were included in the final data analysis. The mean age of enrolled patients was 48.1 years, and 66.9% (107/160) of them were male patients. A total of 41 patients achieved chemotherapeutic resistance with an incidence of 25.6% (41/160). The demographic and clinical characteristics associated with chemotherapeutic resistance in advanced GC patients are summarized in Table 1. The mean age (P = 0.032) and cycles of chemotherapy (P = 0.040) were significantly lower in patients with chemotherapeutic resistance than those without chemotherapeutic resistance. In addition, patients with chemotherapeutic resistance showed significantly larger tumor sizes (P = 0.027). Additionally, current smoking habits (P = 0.013) seemed to be associated with an increased incidence of chemotherapeutic resistance. No statistical differences were observed in BMI, gender, ASA physical status, drinking habits, comorbidities of diabetes and hypertension, ECOG status, types of surgery and surgical approach, operation time, estimated blood loss, chemotherapy regimen, tumor location, tumor differentiation, Lauren’s classification, clinical and pathological TNM stage, or Her-2 status between the two groups (P > 0.05).
The pretreatment laboratory tests associated with chemotherapeutic resistance are summarized in Table 2. In comparison with patients without chemotherapeutic resistance, patients with chemotherapeutic resistance showed significantly lower pretreatment AFR levels (P = 0.001). Additionally, those patients with elevated pretreatment CEA (P = 0.029) and CA19-9 (P = 0.034) levels were more likely to experience chemotherapeutic resistance. The laboratory tests of patients with or without chemotherapeutic resistance were not significantly different in terms of Hb, WBC, platelet, Cr, urea, CA72-4, and CA125 (P > 0.05).
–
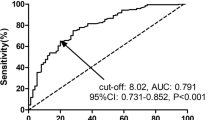
ROC curves were constructed to evaluate the performance of four continuous variables (age, cycles of chemotherapy, tumor size, and AFR) to predict chemotherapeutic resistance (see Fig. 2). Age (cut-off value: 46.5, AUC: 0.617, P = 0.026) and AFR (cut-off value: 10.85, AUC: 0.740, P < 0.001) were two predictors of chemotherapeutic resistance by ROC curve analysis. Based on the cut-off values, these were categorized into high (≥ cut off value) and low (< cut off value) groups.
Thereafter, seven potential risk factors (P < 0.05 in Tables 1 and 2) were included in the univariate logistic regression model. As indicated in Fig. 3, current smoking habits, large tumor size, low AFR, and high CEA were four potential risk factors for chemotherapeutic resistance. After including these four factors in the multivariate logistic regression model, the results revealed that low AFR (OR: 2.55, 95%CI: 1.21–4.95, P = 0.005) was an independent risk factor of chemotherapeutic resistance (Fig. 4). In addition, the results from multivariate COX regression analyses also indicated low AFR as an independent prognostic factor for 5-year OS (HR: 0.36, 95%CI: 0.15–0.73, P = 0.011, see Table 3).
Moreover, we performed Kaplan–Meier curve analysis to evaluate the association between the survival and pretreatment AFR level (see Fig. 5). The study’s results indicated that a low AFR (< 10.85) was associated with a poorer 5-year DFS (Fig. 5A) and OS (Fig. 5B).
Discussion
In this study, the potential risk factors for chemotherapy resistance in GC patients were evaluated. The overall incidence of chemotherapy resistance was calculated to be 25.6%, which was quite similar to the reported 22.4% by Wan et al. [13]. The multivariate analysis indicated that low AFR was an independent risk factor for chemotherapeutic resistance in GC patients. Moreover, patients with a low AFR (< 10.85) tended to have worse clinical outcomes as determined by survival analysis. To our knowledge, this study is the first to highlight the close association between pretreatment AFR, chemotherapeutic resistance, and clinical outcomes. The close association between chemotherapy resistance and prognosis has been widely accepted [14].
It has been previously reported that preoperative AFR was an independent predictor of chemotherapy resistance and prognosis among patients with advanced epithelial ovarian cancer [14]. Additionally, a recent study by Li et al. [15] also suggested the pretreatment AFR level as a novel predictor of chemotherapy response and prognosis in patients with locally advanced rectal cancer after surgery. These findings are in agreement with our results. All these results strongly suggest a close association between AFR and chemotherapy response. A previous study by Zhang et al. [16] indicated the prognostic value of fibrinogen/pre-Albumin ratio (FPR) in patients with surgical stage II and III GC. Although with some differences (e.g. inclusion criteria, biomarkers, and observation endpoints), our study was in accordance with their conclusions.
A meta-analysis by Ma et al. [17], which included six studies, concluded that decreased lymphocyte to monocyte ratio (LMR) was associated with worse OS in GC patients. A previous study indicated that elevated neutrophil to lymphocyte ratio (NLR) correlated with late-stage GC and worse prognosis [18]. Another study suggested that preoperative, postoperative, and changes of NLR levels were all significant prognostic factors in GC patients [19]. Platelet to lymphocyte ratio (PLR), another peripheral blood-derived inflammation marker, has also been reported to be a prognostic factor in advanced GC patients after radical resection [20, 21]. In addition, the combination of NLR and PLR was also recognized as a promising predictor for tumor response and prognosis in advanced GC patients [22, 23]. Dysregulated noncoding RNAs are also involved in the mechanisms of chemotherapy resistance and have the potential to serve as novel therapeutic targets and prognostic biomarkers for GC [24, 25].
It is widely accepted that inflammation is an important contributor to the angiogenesis, proliferation, metastasis, and resistance to hormonal treatments and chemotherapy of tumors [26]. In addition, chemotherapy-induced inflammation is commonly observed in the therapeutic process, and it can lead to tumor-acquired resistance, which often results in treatment failure and tumor metastasis [27]. In patients with cancer, the expression of serum Alb is correlated with an increased tumor-induced inflammatory reaction [28]. Fib can be synthesized by tumor cells [29], and its synthesis can significantly increase in response to ongoing tumor-induced inflammation [30]. Covering both Alb and Fib, AFR can reflect systemic inflammation in patients with amplified sensitivity and effectiveness [14]. Given the very close association between inflammation and chemotherapy resistance, the predictive role of AFR in chemotherapy resistance can be understood and explained. This study indicated pretreatment AFR level as a prognostic factor in 5-year DFS and OS among GC patients. Considering the correlation between prognosis and chemotherapy resistance, the predictive role of AFR for chemotherapy resistance might be a possible explanation for its predictive role of prognosis in GC patients.
In conclusion, this study indicated that a low level of pretreatment AFR could serve as an independent predictor of chemotherapy resistance and postoperative prognosis in GC patients following radical gastrectomy. This study has some limitations. First, this is a retrospective study with relative small sample size. Second, whether pre-surgical AFR can serve as an independent predictor remains unknown. Third, the cut-off value of AFR was calculated based on our own variables and whether it can be generalized needs to be further external validated.
Availability of data and materials
The datasets are available from the corresponding author on reasonable request.
References
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J, et al. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Ann Surg. 2018;267(2):311–8.
Zhou Y, Sun X, Zhou L, Zhang X. pH-Sensitive and long-circulation nanoparticles for near-infrared fluorescence imaging-monitored and chemo-photothermal synergistic treatment against gastric cancer. Front Pharmacol. 2020;11:610883.
Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage iii gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304.
Sawaki K, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. Survival times are similar among patients with peritoneal, hematogenous, and nodal recurrences after curative resections for gastric cancer. Cancer Med. 2020;9(15):5392–9.
Sun DW, An L, Lv GY. Albumin-fibrinogen ratio and fibrinogen-prealbumin ratio as promising prognostic markers for cancers: an updated meta-analysis. World J Surg Oncol. 2020;18(1):9.
Bian NN, Shi XY, Qi HY, Hu X, Ge Y, An GY, et al. The relationship of plasma fibrinogen with clinicopathological stages and tumor markers in patients with non-small cell lung cancer. Medicine (Baltimore). 2019;98(32):e16764.
Zhang Y, Xiao G. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: a meta-analysis. Cancer Manag Res. 2019;11:3381–93.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–55.
Kim IH, Park SS, Lee CM, Kim MC, Kwon IK, Min JS, et al. Efficacy of Adjuvant S-1 Versus XELOX Chemotherapy for patients with gastric cancer after d2 lymph node dissection: a retrospective, multi-center observational study. Ann Surg Oncol. 2018;25(5):1176–83.
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015;26(1):141–8.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9.
Wan J, Chao L, Lee AC, Chen Q. Higher Expression of ERCC1 may be associated with resistance to adjuvant platinum-based chemotherapy in gastric cancer. Cancer Invest. 2017;35(2):85–91.
Yu W, Ye Z, Fang X, Jiang X, Jiang Y. Preoperative albumin-to-fibrinogen ratio predicts chemotherapy resistance and prognosis in patients with advanced epithelial ovarian cancer. J Ovarian Res. 2019;12(1):88.
Li H, Wang H, Shao S, Gu Y, Yao J, Huang J. Pretreatment albumin-to-fibrinogen ratio independently predicts chemotherapy response and prognosis in patients with locally advanced rectal cancer undergoing total mesorectal excision after neoadjuvant chemoradiotherapy. Onco Targets Ther. 2020;13:13121–30.
Zhang J, Li SQ, Liao ZH, Jiang YH, Chen QG, Huang B, et al. Prognostic value of a novel FPR biomarker in patients with surgical stage II and III gastric cancer. Oncotarget. 2017;8(43):75195–205.
Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: a meta-analysis. Int J Surg. 2018;50:67–71.
Sun J, Chen X, Gao P, Song Y, Huang X, Yang Y, et al. Can the neutrophil to lymphocyte ratio be used to determine gastric cancer treatment outcomes? a systematic review and meta-analysis. Dis Markers. 2016;2016:7862469.
Kim EY, Song KY. The preoperative and the postoperative neutrophil-to-lymphocyte ratios both predict prognosis in gastric cancer patients. World J Surg Oncol. 2020;18(1):293.
Gu L, Wang M, Cui X, Mo J, Yuan L, Mao F, et al. Clinical significance of peripheral blood-derived inflammation markers in advanced gastric cancer after radical resection. BMC Surg. 2020;20(1):219.
Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol. 2015;22(13):4363–70.
Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672.
Konopka K, Micek A, Ochenduszko S, Streb J, Potocki P, Kwinta L, et al. Combined neutrophil-to-lymphocyte and platelet-volume-to-platelet ratio (nlr and pvpr score) represents a novel prognostic factor in advanced gastric cancer patients. J Clin Med. 2021. https://doi.org/10.3390/jcm10173902.
Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, et al. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. 2020;19(1):62.
Yuan L, Xu ZY, Ruan SM, Mo S, Qin JJ, Cheng XD. Long non-coding RNAs towards precision medicine in gastric cancer: early diagnosis, treatment, and drug resistance. Mol Cancer. 2020;19(1):96.
Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33(3):127–48.
Simone BA, Palagani A, Strickland K, Ko K, Jin L, Lim MK, et al. Caloric restriction counteracts chemotherapy-induced inflammation and increases response to therapy in a triple negative breast cancer model. Cell Cycle. 2018;17(13):1536–44.
Tuomisto AE, Makinen MJ, Vayrynen JP. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J Gastroenterol. 2019;25(31):4383–404.
Bekos C, Grimm C, Brodowicz T, Petru E, Hefler L, Reimer D, et al. Prognostic role of plasma fibrinogen in patients with uterine leiomyosarcoma - a multicenter study. Sci Rep. 2017;7(1):14474.
Bai J, He A, Huang C, Yang J, Zhang W, Wang J, et al. Serum peptidome based biomarkers searching for monitoring minimal residual disease in adult acute lymphocytic leukemia. Proteome Sci. 2014;12(1):49.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
GJ Z participated in the conception and design, data collection, statistical analysis, wrote the manuscript, the conception and design and data collection.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was approved by the ethic committee of Taizhou People’s Hospital. Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, G. Albumin/fibrinogen ratio, a predictor of chemotherapy resistance and prognostic factor for advanced gastric cancer patients following radical gastrectomy. BMC Surg 22, 207 (2022). https://doi.org/10.1186/s12893-022-01657-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01657-1