Abstract
Background
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) has been introduced for palliative treatment of peritoneal surface malignancies (PSM) and is currently tested also in the neoadjuvant and prophylactic setting. The aim was therefore to compare safety and tolerance of staging laparoscopy with or without PIPAC.
Methods
This retrospective analysis compared consecutive patients undergoing staging laparoscopy alone for oesogastric cancer with patients having PIPAC for suspected PSM of various origins from January 2015 until January 2020. Safety was assessed by use of the Clavien classification for complications and CTCAE for capturing of adverse events. Pain and nausea were documented by use of a visual analogue scale (VAS: 0–10: maximal intensity).
Results
Overall, 25 PIPAC procedures were compared to 24 staging laparoscopies. PIPAC procedures took a median of 35 min (IQR: 25–67) longer. Four patients experienced at least one complication in either group (p = 0.741). No differences were noted for postoperative nausea (p = 0.961) and pain levels (p = 0.156). Median hospital stay was 2 (IQR: 1–3) for PIPAC and 1 (IQR: 1–2) for the laparoscopy group (p = 0.104).
Conclusions
The addition of PIPAC did not jeopardize safety and postoperative outcomes of staging laparoscopy alone. Further studies need to clarify its oncological benefits.
Similar content being viewed by others
Background
Treatment of peritoneal metastases (PM) remains an oncological and surgical challenge [1,2,3,4]. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) has been proposed as a novel method of intraperitoneal drug delivery for patients with peritoneal surface malignancies (PSM), claiming improved distribution, enhanced tissue uptake, better tolerance and repeatability using minimally invasive access [5, 6]. Recent systematic reviews confirmed PIPAC to be a safe and promising treatment option for patients with unresectable advanced isolated peritoneal disease, refractory to systemic treatment [7, 8]. Objective clinical response was reported in the palliative setting in 62–88% of patients with ovarian cancer, between 50 and 91% for gastric cancer and 71–86% for colorectal cancer [8]. PIPAC combined with systemic chemotherapy was also recently suggested as neoadjuvant treatment, in an attempt to make initially non-resectable patients eligible for secondary CRS and HIPEC with curative intent [9, 10]. Hence, it appears reasonable to consider PIPAC in the neoadjuvant and prophylactic setting while performing the initial staging laparoscopy in patients at high risk for presence of microscopic deposits or development of metachronous PSM [11].
The aim of this study was to assess safety and tolerance of the addition of PIPAC to baseline staging laparoscopy, for patients with high-risk features for PSM.
Methods
This single centre retrospective comparative study included all consecutive patients admitted for staging laparoscopy, during workup of intra-abdominal neoplasia of various origin (colorectal, appendicular, gastric and ovarian). Indications for adding PIPAC were either suspected PSM on baseline imaging or a high-risk constellation. Indications for the procedure were decided in the multidisciplinary tumor board and all patients signed informed consent. The study period lasted from January 2015 (start of PIPAC program in our department) to January 2020. Patients in the PIPAC group were compared to all consecutive patients with laparoscopic staging alone (laparoscopy group)) for gastro oesophageal junction (GOJ) adenocarcinoma (Siewert II and III) classified uT3 or uT4 [12]. Baseline demographics were compared according to the age, gender, BMI (kg/m2), ASA score and Charlson Comorbidity Index (CCI) [13]. Staging laparoscopy with peritoneal washing was performed according to the current ESMO guidelines for all patients with resectable stage IB-III gastric adenocarcinoma, to exclude the presence of occult peritoneal carcinomatosis [14]. No prophylactic PIPAC was foreseen in this setting without suspected PSM on baseline imaging, and as a consequence no PIPAC could be delivered for patients with intraoperative diagnosis of peritoneal implants. Staging laparoscopy was uniformly performed in both groups, with systematic assessment of all abdominal regions according to Sugarbaker's peritoneal cancer index (PCI) [15]. All laparoscopies in both groups (laparoscopy alone and PIPAC) were performed before resection of primary. Criteria of exclusion was age < 18 years old and patients’ refusal to participate.
Outcomes
Safety, tolerance and potential chemotherapy-related adverse events were assessed by documentation of postoperative complications according to the Clavien classification and by use of CTCAE v5.0 [16, 17]. Nausea and postoperative pain (at rest) were measured on routine basis by use of a visual analogue scale (0–10: maximal intensity) 3x/d.
Data Management
Demographics, oncological and surgical data were retrieved from a prospectively maintained institutional database and entered in an a priori defined anonymized data base. The following variables were extracted: age, gender, primary tumour origin, body mass index, ASA class, Charslon Comorbidity Index (CCI) [13], intra-abdominal chemotherapy regimen (for PIPAC), PCI (Peritoneal Cancer Index), overall postoperative complications (according to Calvien-Dindo), postoperative pain and nausea (VAS- visual analog scale: 0–10). Analgesia protocols were comparable between the two groups without use of opioids only on demand.
PIPAC procedure and safety considerations
PIPAC procedure has been detailed previously and was applied according to current recommendations and safety protocols [18]. Oxaliplatin was applied at a dose of 92 mg/m2 for carcinosis of colorectal origin. Cisplatin (7.5 mg/m2) in combination with Doxorubicin (1.5 mg/m2), with dose adaptation since 2019 according to Tempfer’s phase 1 trial (10.5 mg/m2 and 2.1 mg/m2) was used for ovarian, gastric, and other malignancies [19]. Aerosol chemotherapy was applied using electrostatic precipitation (ePIPAC) in our department since 2017 [20].
Statistics and analysis
Continuous variables were presented as mean with standard deviation (SD) or median with interquartile range (IQR) according to their distribution. Categorical variables were reported as frequencies (%) and compared with chi-square test. Student t-test or Mann–Whitney test were used to compare continuous variables. A linear mixed-effect model to assess the effect of surgery type on VAS scores, when correcting for time. All statistical tests were two-sided and a level of 0.05 was used to indicate statistical significance. Statistical analyses were performed with GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA, USA).
Ethics
The study was approved by local Commission on Ethics in Human Research (CER-VD 2019–00747) and was conducted in compliance with the current version of the STROBE statement (www.strobe-statement.org) [21].
Results
Forty-nine patients (M: F = 32: 17, mean age 60 ± 11 years) underwent during the study period either laparoscopy alone (LA) (n = 24) or laparoscopy + PIPAC (LP) (n = 25) group. LP group included 10 patients with colorectal primary (42%), 6 gastric (22%), 5 ovarian (20%) and 4 appendicular (16%). Median PCI in the LP group was 10 (Range: 0–22). Five patients (20%) in the LP had no macroscopic disease (PCI = 0), one of these five patients had positive cytology. No patient had concomitant IV chemotherapy during PIPAC procedure. All patients in the LA group had GOJ adenocarcinoma (16 uT3 (66%) and 8 uT4 (34%)), 3 of them had PC (13%) two with a PCI 3 one PCI 15 and one patient had positive cytology with a PCI of 0. Median surgical time (p = 0.001) and number of trocars were significantly different between the two groups (p = 0.011). There were no intraoperative complications in any of the 49 procedures. Baseline demographics and surgical details are displayed in Table 1.
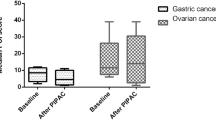
There was no significant difference between LA vs. LP regarding length of stay, postoperative nausea and overall complications (Table 2). Post-operative complications were: 2 subcutaneous hematoma, 1 urinary retention, 1 ileus requiring nasogastric tube (NGT) in the LA group and 3 subcutaneous hematomas [1 requiring transfusion] and 1 urinary retention in the LP group. No postoperative complications were directly related to intra-peritoneal chemotherapy in the LP group. No difference was found between the two groups regarding post-operative pain. (Fig. 1).
Discussion
In this study, the combination of staging laparoscopy with PIPAC was equally safe and well tolerated as staging laparoscopy alone. Surgery time was longer in the LP group, but early clinical outcomes and hospital length of stay were similar. The potential benefits of added PIPAC remain yet to be investigated.
Preliminary published studies have shown good tolerance and safety of PIPAC [7, 8]. However, these studies have been carried out for the most part in palliative situations. This study investigates the effect of PIPAC in a neoadjuvant/prophylactic setting and aimed the tolerance of PIPAC to staging laparoscopy alone. The results of this preliminary study are encouraging and support further evaluation of PIPAC in a neoadjuvant setting.
PSM comprises a heterogeneous group of quite different primaries. Most frequent origins spreading within the abdominal cavity at initial presentation are ovarian (46%), oesogastric (14%), and colorectal tumors (5%) [1, 2, 4]. Staging laparoscopy for primary digestive malignancies allows identification of occult peritoneal disease [22, 23]. According to ESMO guidelines, staging laparoscopy is recommended for all patients with locally advanced gastro oesophageal adenocarcinoma (> cT3 and/or cN + stage) [14]. In particular, tumors that develop within the abdominal cavity (Siewert II and III) are more susceptible to present a peritoneal metastatic spread (6%–17%) upon initial diagnosis [24, 25]. Hence, diagnostic laparoscopy is an integral part of locally advanced gastric and gastroesophageal junction cancer staging [24]. Occult PSM precluding upfront curative surgery is discovered in 15 to 40% of patients with locally advanced gastric cancer during staging laparoscopy [26,27,28]. ESMO guidelines recommend a staging laparoscopy in all potentially resectable stage IB–III gastric tumors, [14] whereas the SAGES guidelines recommend staging laparoscopy for T3/T4 gastric cancer without evidence of lymph node or distant metastasis on high quality preoperative imaging [23]. In colorectal cancer, approximately 20–30% of patients with pT4 or perforated tumors develop metachronous peritoneal metastases, but staging laparoscopy is not performed systematically [29, 30]. In this situation, primary tumor resection is often necessary even in presence of PM to treat or even to prevent imminent obstruction, perforation or bleeding [23]; as such, the additional value of staging laparoscopy in the primary and metastatic colorectal cancer has not yet been clearly defined [31, 32]. However, 10% of occult peritoneal metastases for pT4 cancer are diagnosed during a planned second look laparoscopy 6 months after resection, even when no metastases are detected on high-resolution abdominal imaging [29]. For appendiceal tumors, the detection rate of PM is up to 23% at one year after primary resection for mucinous neoplasm. Thus, laparoscopy was suggested as a primary screening tool during postoperative follow-up, to identify occult metastases undetectable on CT scan [33]. In the context of ovarian epithelial cancer, staging laparoscopy was suggested as a routine in the initial diagnostic workup [34], leading to upstaging of the disease through detection of PM in 22.6% of patients [35].
Prognosis of patients with advanced PM is dismal and depends mainly on disease extent and response to therapy [7]. Resistance to systemic chemotherapy due to limited drug distribution in the peritoneum and limitations in early-stage disease diagnosis with non-invasive imaging make PM management particularly challenging [3].
Complete abdominal surgical exploration in high-risk patients with PM has been described and evaluated in prospective non-randomised studies [36,37,38,39,40]. Similarly, a systematic second look is proposed for early diagnosis of peritoneal metastases from colorectal origin, not visible on imaging. This second-look allows early treatment of patients with low PCI; although this may improve survival in selected cases, it rarely allows a curative treatment strategy. To overcome this problem intraabdominal chemotherapy was evaluated as adjuvant treatment in cohort studies [41,42,43]. These preliminary studies reported promising results of adjuvant HIPEC for high-risk colon cancer, decreasing the incidence of peritoneal metastasis to 0–4% after IntraPeritoneal treatment. Five randomized studies aimed to determine the efficacy of adjuvant HIPEC in patients with locally advanced colon cancer: PROPHYLOCHIP–PRODIGE 15 trial [44], COLOPEc trial [30], APEC Study [45], HIPECT4 trial [46] and PROMENADE trial (47). Systematic second-look surgery plus oxaliplatin-HIPEC did not improve disease-free survival compared to standard surveillance in ROPHYLOCHIP–PRODIGE 15 and COLOPEc, but was related to up to 41% of postoperative complications (grade 3–4) [30, 44]. HIPECT4 trial observed a reduced risk of peritoneal recurrence from 36 to 18% at 36 months for T4 colon-rectal carcinoma after adjuvant HIPEC [46]. Results of APEC and PROMENADE trials are still awaited [45, 47].
Pressurised intraperitoneal aerosol chemotherapy (PIPAC) has been proposed as an alternative mode for intraperitoneal drug delivery in certain situations, claiming improved distribution, enhanced tissue uptake, better tolerance, and repeatability using minimally invasive access [5, 6, 8]. Favourable initial reports [7] have triggered the adoption of PIPAC as a drug delivery technique. PIPAC has been proposed as an alternative method of intraperitoneal drug delivery, claiming improved distribution, enhanced tissue uptake, better tolerance and repeatability using minimally invasive access [5, 6, 8]. In recent systemic reviews, PIPAC is considered a safe and promising treatment alternative for patients with advanced isolated refractory peritoneal disease [7, 8].
Adjuvant PIPAC for high-risk patients is an intriguing concept which entails a risk of overtreatment. Added PIPAC in this study did not increase the risk or tolerance of staging laparosocopy alone. On the other hand, the risk of missed opportunities (no PIPAC with patients with positive cytology) is to consider and might favour local spread. Three prospective randomized trials are currently recruiting to assess PIPAC as adjuvant treatment: the GASPACCO [48] and PIPAC-OPC4 [49] trials for T3-4 Gastric Cancer and the PIPAC-OPC3 CC trial for high risk colon cancer [50].
Following the principles of the IDEAL framework allowing standardized approach, future prospective studies are needed to confirm the efficacy and oncologic benefits of PIPAC [51]. Furthermore, a registry for quality control supported by the International Society for the Study of Pleura and Peritoneum (ISSPP) was launched in 2020. This international database hosted at the University of Odense will facilitate future research with prospective monitoring [52].
The current study has some limitations which are mainly related to its retrospective nature and limited patient number and heterogeneity of groups. Differences between the comparative groups might have passed undetected due to type II error. Although baseline characteristics of patients were comparable there was no random allocation for the two groups with a consequent risk for selection bias. Arguably, patients in the PIPAC group might have been a higher risk for complications due to more advanced peritoneal disease (higher PCI) and prior treatments. This report could therefore be interpreted as indirect confirmation of the safety of PIPAC (i.e. same complication rates in "worse" patients). Even if the number of patients was low in both arms with heterogeneous patients, the groups were comparable for ASA score and only slightly different regarding the Charlson Comorbidity Index score. Furthermore, both comparative groups were treated by the same surgical team in the same hospital and with the same perioperative care strategies.
Conclusions
In conclusion, neoadjuvant and prophylactic PIPAC could be proposed to patients undergoing staging laparosocopy for suspected or confirmed PSM with minimal increase in surgery time, but no increase in risk and tolerance of the procedure. Its oncological efficacy in this context is currently investigated under controlled conditions. Until then, PIPAC should only be performed in expert centers under standardized conditions and with and prospective monitoring and systematic patient follow-up.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PIPAC:
-
Pressurized IntraPeritoneal Aerosol Chemotherapy
- PSM:
-
Peritoneal surface malignancy
- PCI:
-
Peritoneal Cancer Index
- PM:
-
Peritoneal metastasis
References
Henderson JT, Webber EM, Sawaya GF. Screening for ovarian cancer: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;319(6):595–606.
Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128(11):2717–25.
Sasson AR, Kim J. Many challenges of peritoneal carcinomatosis. J Oncol Pract. 2017;13(7):435–6.
Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134(3):622–8.
Solass W, Giger-Pabst U, Zieren J, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol. 2013;20(11):3504–11.
Solass W, Kerb R, Murdter T, Giger-Pabst U, Strumberg D, Tempfer C, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21(2):553–9.
Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Hubner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg. 2017;104(6):669–78.
Alyami M, Hubner M, Grass F, Bakrin N, Villeneuve L, Laplace N, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20(7):e368–77.
Girshally R, Demtroder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2016;14(1):253.
Alyami M, Gagniere J, Sgarbura O, Cabelguenne D, Villeneuve L, Pezet D, et al. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43(11):2178–83.
Graversen M, Detlefsen S, Fristrup C, Pfeiffer P, Mortensen MB. Adjuvant Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in resected high-risk colon cancer patients - study protocol for the PIPAC-OPC3 Trial. A prospective, controlled phase 2 Study. Pleura Peritoneum. 2018;3(2):20180107.
Rudiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232(3):353–61.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38–49.
Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–74.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
Hubner M, Grass F, Teixeira-Farinha H, Pache B, Mathevet P, Demartines N. Pressurized intraperitoneal aerosol chemotherapy - practical aspects. Eur J Surg Oncol. 2017;43(6):1102–9.
Tempfer CB, Giger-Pabst U, Seebacher V, Petersen M, Dogan A, Rezniczek GA. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol. 2018;150(1):23–30.
Reymond M, Demtroeder C, Solass W, Winnekendonk G, Tempfer C. Electrostatic precipitation Pressurized IntraPeritoneal Aerosol Chemotherapy (ePIPAC): first in-human application. Pleura Peritoneum. 2016;1(2):109–16.
STROBE Statement: https://www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_case-control.pdf.
Blackshaw GR, Barry JD, Edwards P, Allison MC, Thomas GV, Lewis WG. Laparoscopy significantly improves the perceived preoperative stage of gastric cancer. Gastric Cancer. 2003;6(4):225–9.
Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Guidelines for diagnostic laparoscopy. Reviewed and approved in April 2010. https://www.sages.org/publications/guidelines/guidelines-for-diagnostic-laparoscopy/ Accessed 5 May 2021.
Strandby RB, Svendsen LB, Ambrus R, Rostved AA, Hasselby JP, Achiam MP. The incidence of free peritoneal tumor cells before and after neoadjuvant chemotherapy in gastroesophageal junction cancer. J Cytol. 2020;37(1):40–5.
Convie L, Thompson RJ, Kennedy R, Clements WD, Carey PD, Kennedy JA. The current role of staging laparoscopy in oesophagogastric cancer. Ann R Coll Surg Engl. 2015;97(2):146–50.
de Graaf GW, Ayantunde AA, Parsons SL, Duffy JP, Welch NT. The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol. 2007;33(8):988–92.
Borgstein ABJ, van Henegouwen MI, Lameris W, Eshuis WJ, Gisbertz SS, Dutch Upper GICA. Staging laparoscopy in gastric cancer surgery. A population-based cohort study in patients undergoing gastrectomy with curative intent. Eur J Surg Oncol. 2020. 17(4):1041
Machairas N, Charalampoudis P, Molmenti EP, Kykalos S, Tsaparas P, Stamopoulos P, et al. The value of staging laparoscopy in gastric cancer. Ann Gastroenterol. 2017;30(3):287–94.
Bastiaenen VP, Klaver CEL, Kok NFM, de Wilt JHW, de Hingh I, Aalbers AGJ, et al. Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases; the COLOPEC 2 randomized multicentre trial. BMC Cancer. 2019;19(1):254.
Klaver CEL, Wisselink DD, Punt CJA, Snaebjornsson P, Crezee J, Aalbers AGJ, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(10):761–70.
Cotte E, Peyrat P, Piaton E, Chapuis F, Rivoire M, Glehen O, et al. Lack of prognostic significance of conventional peritoneal cytology in colorectal and gastric cancers: results of EVOCAPE 2 multicentre prospective study. Eur J Surg Oncol. 2013;39(7):707–14.
Mohan HM, O’Connor DB, O’Riordan JM, Winter DC. Prognostic significance of detection of microscopic peritoneal disease in colorectal cancer: a systematic review. Surg Oncol. 2013;22(2):e1-6.
Kelly KJ. Management of Appendix Cancer. Clin Colon Rectal Surg. 2015;28(4):247–55.
Zeff N. Role of laparoscopy in initial tumour staging in advanced epithelial ovarian cancer: a systematic review. Pleura Peritoneum. 2018;3(1):20180106.
Park HJ, Kim DW, Yim GW, Nam EJ, Kim S, Kim YT. Staging laparoscopy for the management of early-stage ovarian cancer: a metaanalysis. Am J Obstet Gynecol. 2013;209(1):58.
Elias D, Honore C, Dumont F, Ducreux M, Boige V, Malka D, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254(2):289–93.
Delhorme JB, Triki E, Romain B, Meyer N, Rohr S, Brigand C. Routine second-look after surgical treatment of colonic peritoneal carcinomatosis. J Visc Surg. 2015;152(3):149–54.
Passot G, Dumont F, Goere D, Arvieux C, Rousset P, Regimbeau JM, et al. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br J Surg. 2018;105(6):663–7.
Cortes-Guiral D, Elias D, Cascales-Campos PA, Badia Yebenes A, Guijo Castellano I, Leon Carbonero AI, et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy for patients with colorectal cancer at high risk of peritoneal carcinomatosis: does it really save lives? World J Gastroenterol. 2017;23(3):377–81.
Moral ASD, Viejo EP, Romero IM, Perez FP. Results of systematic second-look surgery plus hipec in perforated or pt4 colon cancer. Case series. Ann Med Surg. 2021;62:386–90.
Sammartino P, Sibio S, Biacchi D, Cardi M, Accarpio F, Mingazzini P, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. Gastroenterol Res Pract. 2012;2012:141585.
Tentes AA, Spiliotis ID, Korakianitis OS, Vaxevanidou A, Kyziridis D. Adjuvant perioperative intraperitoneal chemotherapy in locally advanced colorectal carcinoma: preliminary results. ISRN Surg. 2011;2011:529876.
Chouillard E, Ata T, De Jonghe B, Maggiori L, Helmy N, Coscas Y, et al. Staged laparoscopic adjuvant intraperitoneal chemohyperthermia after complete resection for locally advanced colorectal or gastric cancer: a preliminary experience. Surg Endosc. 2009;23(2):363–9.
Tanis PJ, Tuynman JB, de Hingh I. Results from the PROPHYLOCHIP-PRODIGE 15 trial. Lancet Oncol. 2020;21(11):e496.
https://clinicaltrials.gov: Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Versus no HIPEC in Locally Advanced Colorectal Cancer (APEC Study): NCT02965248.
Arjona-Sanchez A, Barrios P, Boldo-Roda E, Camps B, Carrasco-Campos J, Concepcion Martin V, et al. HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer. 2018;18(1):183.
https://clinicaltrials.gov: Proactive Management of Endoperitoneal Spread in Colonic Cancer (PROMENADE): NCT02974556.
https://clinicaltrials.gov: Benefits of Pressured Intraperitoneal Aerosol Chemotherapy (PIPAC) in Patients With T3-4 Gastric Cancer Cyt- (GASPACCO): NCT04595929.
https://clinicaltrials.gov: Adjuvant PIPAC in Gastric Cancer Patients (PIPAC-OPC4): NCT04047004.
ttps://clinicaltrials.gov: Adjuvant Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in Resected High Risk Colon Cancer Patients: NCT03280511.
Tate SJ, Torkington J. Pressurized intraperitoneal aerosol chemotherapy: a review of the introduction of a new surgical technology using the IDEAL framework. BJS Open. 2020;4(2):206–15.
Mortensen MB, Glehen O, Horvath P, Hübner M, Hyung-Ho K, Königsrainer A, et al. The ISSPP PIPAC database: design, process, access, and first interim analysis. Pleura Peritoneum. 2021;6(3):91–7.
Acknowledgements
This original work was presented as a poster at the second ISSPP (International Society for the Study of Pleura and Peritoneum) congress on October 7th – 8th, 2021 in Roma, Italy. The abstract is published in the ISSPP journal Pleura and Peritoneum (https://www.degruyter.com/document/doi/10.1515/pp-2021-0141/html).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
HTF, DM, SM, ND & MH meet all the criteria for the definition of authorship and contributed substantially to the manuscript. HTF & MH conceived and designed the study. HTF, DM & SM managed the data and analyzed the data. MH is the corresponding author. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki. Informed written consent was obtained for all participants and the study was approved by the Institutional Review Board (or Ethics Committee) of canton Vaud, Switzerland: CER-VD (CER-VD 2019–00747).
Consent for publication
Not applicable.
Competing interests
Hugo Teixeira-Farinha, Daphné Mattille, Styliani Mantziari, Nicolas Demartines and Martin Hübner declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Teixeira Farinha, H., Mattille, D., Mantziari, S. et al. Early postoperative outcomes of staging laparoscopy for peritoneal metastases with or without pressurized intra-peritoneal aerosol chemotherapy (PIPAC). BMC Surg 22, 122 (2022). https://doi.org/10.1186/s12893-022-01572-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-022-01572-5