Abstract
Background
Congenital pericardial defects are rare congenital anomalies, often asymptomatic and incidentally detected during thoracic surgery.
Case presentation
A 74-year-old man with primary lung cancer (cT1cN0M0, Stage IA3) underwent thoracoscopic radical lobectomy. At the time of thoracotomy, a pericardial defect was found on the ventral side of the hilar region, and the left atrial appendage was exposed. Due to concern that contact between the bronchial stump and the left atrial appendage may lead to postoperative bleeding and heart hernia, the pericardial defect was closed with an expanded polytetrafluoroethylene GoreTex® membrane. Preoperative computed tomography was reanalyzed with a 1 mm slice, congenital pericardial defect was detected as the pericardium had penetrated under the left atrial appendage.
Conclusions
In congenital partial pericardial defect, contact between the left atrial appendage and bronchial stump, due to movement of the lung or heart, increases the risk of bleeding after lung resection. Therefore, closure of the defect should be considered. Although it is difficult to diagnose congenital partial pericardial defect preoperatively, computed tomography taken with a slice thickness of 1 mm is useful for diagnosis.
Similar content being viewed by others
Background
Congenital pericardial defect (CPD) is a rare anomaly that refers to congenital absence of the pericardium. It can occur as a complete absence of the entire pericardium, the right or left portion of the pericardium or a partial, foramen-like defect of the right or left pericardium [1]. The frequency of its occurrence is reported to be 0.01–0.04% [1, 2]. Repair of congenital partial pericardial defects (CPPD) is controversial. Generally, if there are no symptoms due to a pericardial defect, surgical treatment is not required [3]. On the other hand, lobectomy or pneumonectomy reduce the support to the mediastinal organs and can cause cardiac arrest or myocardial ischemia due to cardiac hernia [4, 5]. Therefore, closure of the defect should be considered. Diagnosing CPPD preoperatively is associated with reduced risk of cardiac injury [3]. In pulmonary surgery, complications of pericardial emphysema secondary to pneumothorax are often associated with preoperative diagnosis of CPPD. We report a case of a CPPD that was incidentally diagnosed during surgery for upper left lobe lung cancer, and describe the use fulness of 1 mm sliced CT for the diagnosis of CPPD.
Case presentation
Written, informed consent was obtained from the patient for the publication of this report and its accompanying images.
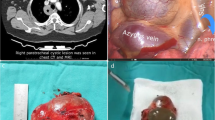
A 74-year-old man was referred because a 32 mm-sized ground glass shadow was pointed out in the anterior segment (S3) of the left lung on chest CT taken for the purpose of medical checkup during follow-up for hypertension and renal dysfunction. He was asymptomatic and had a history of benign prostate hyperplasia with no history of heart disease. Physical findings were normal. Blood urea nitrogen was 24 mg/dL, creatinine was 1.52 mg/dL, and estimated glomerular filtration rate was 35.7 mL/min/1.73 m2. Chest radiography were normal. Non-contrast chest CT showed a ground glass shadow with a maximum tumor diameter of 32 mm and a solid component with a diameter of 24 mm in the left lung S3 (Fig. 1A). No abnormal findings could be pointed out in the cardiac shadow (Fig. 1B). There was no significant hilar or mediastinal lymphadenopathy. 2-deoxy-2-(18F)-fluorodeoxyglucose positron emission tomography exhibited tracer accumulation in the nodules. No accumulation suggesting regional lymph node metastasis or distant metastasis was observed. Atrial fibrillation (Af) and complete right bundle brunch block (RBBB) were observed on the electrocardiogram (ECG). Echocardiography showed that the cardiac function was maintained but the left atrium was enlarged (51 mm). Hypokinesia was observed in the apex, anterior wall, and septum. A decrease in blood flow in the left atrial appendage was observed. The surgery was performed under four port video assisted thoracic surgery. At the time of thoracotomy, the left atrial appendage (LAA) was exposed in the hilar region. Approximately 6.0 × 5.0 cm oval pericardial defect was visualized (CPPD) (Fig. 2A). The phrenic nerve ran on the ventral side of the defect. No pleural effusion or dissemination was observed. Partial resection of the upper lobe of the left lung was performed, and adenocarcinoma was diagnosed by rapid intraoperative diagnosis. Left upper lobectomy and lymph node dissection (ND2a-1) were subsequently performed. We were concerned about cardiac hernia (CH) due to decreased cardiac bearing capacity owing to decreased lung volume and bleeding due to the contact between the LAA and bronchial stump (BS). The BS was covered with pericardial fat pad and the pericardial defect was closed with a 0.1 mm expanded polytetrafluoroethylene (ePTFE) GoreTex® surgical pericardial membrane taking care not to involve the phrenic nerve (Fig. 2B, C). In addition, the pulmonary ligaments were dissected up to the lower edge of the inferior pulmonary vein to reduce upper lobe space. The operation time was 172 min, and the patient lost little blood. Postoperative pathological examination revealed primary lung adenocarcinoma cT1miN0M0, Stage IA1. The postoperative course was uneventful. The chest drainage tube was removed on the 1st postoperative day. Edoxaban was started for Af. He was discharged 5 days after surgery, and remains well at 6-month postoperative follow up.
Intraoperative findings. A The left atrial appendage (LAA) was exposed in front of the hilar region (asterisk). The phrenic nerve was seen on the ventral side of the defect (arrow). B After left upper lobectomy and lymph node dissection (ND2a-1). C The pericardial defect was closed with a 0.1 mm expanded polytetrafluoroethylene GoreTex® surgical pericardial membrane
Discussion and conclusion
CPD is a relatively rare congenital anomaly. Perna’s theory that premature regression of the ducts of Cuvier (common cardinal vein) during the embryonic period delays the growth of thoracic pericardial folds and does not close the thoracic pericardial foramen is predominant [6]. The male–female ratio is 3:1 and is more common in males [1], and 30% are said to have congenital anomalies in the cardiopulmonary system [2]. Of CPD, about 9% were congenital complete pericardial defects (CCPD), 70% were left-side defects (CCPD and CPPD were similar), and 4–6% were right-side defects (mostly CPPD and rarely CCPD). Diaphragmatic pericardial defects accounted for 17%, which is overwhelmingly on the left side [7].
Some cases present with symptoms such as chest pain, palpitation, dyspnea, and absence seizures. However, it is generally asymptomatic and is often found incidentally during surgery [3]. Japanese and English literature regarding CPD found during pulmonary surgery published over 40 years from 1982 to 2021 are shown (Table 1) [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. There have been 32 cases (15 cases of pneumothorax, 7 cases of lung cancer, 5 cases of bronchogenic cyst, and 5 cases of others) including this case, 28 in males and 4 in females. There were 20 cases of CCPD and 12 cases of CPPD with 30 cases on the left side, 1 case on the right side, and 1 case involving both sides. All left-sided CPPDs were found on the upper ventral side of the hilar region. In cases with pneumothorax, it was possible to diagnose a partial pericardial defect preoperatively due to the development of pericardial emphysema as a complication of pneumothorax.
In cases of CCPD, pneumothorax surgery, which is considered to have a small amount of lung resection, does not show left deviation of the heart. However, left-sided deviation of the heart was observed in cases of left upper lobectomy and giant lung cyst resection. Takizawa et al. reported a case in which the coronary circumflex branch stenosis and angina symptoms appeared on the fiber bundle of the pericardial defect due to the leftward deviation of the heart after cutting the giant pulmonary cyst [12], and Honda et al. reported a case of sudden death due to excessive cardiac deviation due to repositioning after performing left upper lobe resection for an inflammatory bronchogenic cyst [4]. These cases are thought to be caused by weakened cardiac support due to decreased lung volume, and caution should be exercised in lobectomy or surgery with a similar amount of resection.
If a partial pericardial defect is found intraoperatively, there is a high risk of sudden death due to CH or pericardial restriction, and repair is necessary [21, 22]. Gassner et al. Argue that cases of death due to partial pericardial defect are due to constriction of the apex of the heart, and that incarceration does not result in death if the defect is localized to the left atrial appendage [23]. However, there are reports that the phrenic nerve was compressed by the deviation of the left atrial appendage without incarceration, causing phrenic nerve paralysis [22]. In this case, the phrenic nerve ran in front of the defect as in previous reports [8, 10]. Therefore, due to the decrease in lung volume due to lobectomy, the incarceration and protrusion of the LAA may be exacerbated and the phrenic nerve may be compressed. What is more worrisome is that movement of the organs may cause contact between the BS and the LAA and lead to perforation and bleeding, so we closed the defect. There were five reports of treatment of these defect, two defects were repaired with artificial membrane, two were repaired with pericardial fat pad (PFP), and one with visceral pleura. Of the 11 CPPD cases reported in the past, all 9 cases with size descriptions had a maximum diameter of 5 cm or less. Three of these patients underwent defect closure. In this case, PFP was used between the pulmonary artery and the BS in order to prevent perforation and bleeding due to contact. Therefore, sufficient PFP could not be collected and the defect was closed with an ePTFE GoreTex® membrane. The ePTFE membrane is known to have no adhesions to the sternum or epicardium during reoperation of cardiac surgery [24]. There is no significant difference in the risk of postoperative mediastinitis when comparing between the group reconstructed with ePTFE membrane and the group reconstructed with autologous tissue [25]. Therefore, when sufficient autologous tissue cannot be obtained, ePTFE is considered useful for pericardial reconstruction.
Recent advances in CT and MRI resolution have made it possible to make q definitive preoperative diagnoses. In a typical case, chest CT shows a finding that lung tissue intervenes between the aorta and the main trunk of the pulmonary artery due to a rotational protrusion to the left lateral side of the pulmonary artery, and a defect in the pericardium in the coronal section or horizontal section [1]. Other findings include right axis deviation and RBBB on ECG, and echocardiography shows enlargement of the right ventricular cavity and abnormal movement of the posterior wall of the left ventricle and the interventricular septum. These findings can lead to a suspicion of CPD [1]. In our case, after the surgery, we reconstructed the preoperative CT with 1 mm slices and found that the pericardium had penetrated under the LAA (Fig. 3). The thickness of the combined visceral and parietal pericardium is usually < 2 mm. Therefore, to accurately resolve the pericardium, an in-plane resolution of at least 1 mm is necessary [1]. It is not reasonable to take a 1 mm sliced CT for all lung surgery cases. However, there is no doubt that CPD should have been suspected on the basis of the ECG and echocardiography.
Preoperative CT reconstructed to a thickness of 1 mm. A, B Coronal section, C Sagittal section, D Horizontal section. Asterisk indicates the left atrial appendage. The white arrow head indicates the pericardium. The white square in (A) is the same range as in (B). The pericardium is seen slipping under the left atrial appendage
In conclusion, we describe a CPPD that was incidentally diagnosed during surgery for left upper lobe lung cancer. In CPPD, contact between the LAA and BS, due to movement of the lung or heart, increases the risk of bleeding after lung resection. Therefore, closure of the defect should be considered. Although it is difficult to diagnose CPPD preoperatively, CT taken with a slice thickness of 1 mm is useful for diagnosis.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- CPD:
-
Congenital pericardial defect
- CPPD:
-
Congenital partial pericardial defects
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- Af:
-
Atrial fibrillation
- RBBB:
-
Right bundle brunch block
- ECG:
-
Electrocardiogram
- LAA:
-
Left atrial appendage
- CH:
-
Cardiac hernia
- BS:
-
Bronchial stump
- ePTFE:
-
Expanded polytetrafluoroethylene
- CCPD:
-
Congenital complete pericardial defects
References
Lopez D, Asher CR. Congenital absence of the pericardium. Prog Cardiovasc Dis. 2017;59:398–406.
Van Son JA, Danielson GK, Schaff HV, Mullany CJ, Julsrud PR, Breen JF. Congenital partial and complete absence of the pericardium. Mayo Clin Proc. 1993;68:743–7.
Siikawa M, Nakanishi K, Endo M, Shiono S. Congenital defect of the pericardium incidentally found during surgery for lung cancer: report of a case. Kyoubu-geka. 2021;74:308–12. https://doi.org/10.15106/j_kyobu74_308 (in Japanese with English abstract).
Honda M, Endo Y, Inoue H. A case of congenital complete absence of left pericardium. J Jpn Surg Assoc. 1985;46:1090–5 (in Japanese with English abstract).
Hanaoka J, Fujino S, Inoue S, Sawai S, Kontani K. A case of congenital pericardial defect found at operation for primary lung cancer. Jpn J Chest Surg. 2000;14:68–72 (in Japanese with English abstract).
Shimada Y, Yoshida J, Aokage K, Hishida T, Nishimura M, Nagai K. Complete left-sided pericardial defect in a lung cancer patient undergoing pneumonectomy without closure of the defect. Ann Thorac Cardiovasc Surg. 2011;17:67–70.
Yamaguchi A, Yoshida S, Ito T. Cardiac displacement after lobectomy in a patient with a congenital complete left-sided pericardial defect. Jpn J Thorac Cardiovasc Surg. 2001;49:317–9.
Uematsu S, Niiya Y, Minakata T, Himuro N, Endo T, Takei H. A case of left spontaneous pneumothorax with patch closure for congenital pericardial defect. Jpn J Chest Surg. 2021;35:107–13 (in Japanese with English abstract).
Siikawa M, Nakanishi K, Endo M, Shiono S. Spontaneous pneumothorax with a congenital defect of the pericardium and hyperthyroidism: report of a case. Jpn J Chest Surg. 2021;35:308–12. https://doi.org/10.2995/jacsurg.35.132 (in Japanese with English abstract).
Date N, Komatsu T, Fujinaga T. Congenital partial pericardial defect confirmed based on spontaneous pneumothorax: a case report and literature review. Int J Surg Case Rep. 2020;75:227–30.
Loo GH, Ismail H, Ismail MI, Md Ali NAB, Abdul Rahman MRB, Haron H. Incidental finding of congenital pericardial defect during vats bullectomy. Tips and tricks to avoid blunder. Ann Med Surg (Lond). 2021;69:102806. https://doi.org/10.1016/j.amsu.2021.102806.
Takizawa H, Ishikura H, Kimura S, Yuasa Y, Okitsu H, Sakata A. Giant pulmonary cyst associated with congenital pericardial defect. Gen Thorac Cardiovasc Surg. 2007;55:65–8.
Ikeda N, Iwanami H, Narita K, Shinohara Y, Tachibana M, Sakonji M, et al. Congenital complete left pericardial defect and giant bulla of the lung repaired surgically: a case report. Jpn J Chest Surg. 1991;5:88–93 (in Japanese with English abstract).
Nakamura T, Sumitomo S, Shaw JB, Taki T, Mitsuoka A, Tamura K, et al. A case of congenital left pericardial defect associated with bilateral nullous emphysema. Jpn J Thorac Dis. 1984;22:1142–6. https://doi.org/10.11389/jjrs1963.22.1142 (in Japanese with English abstract).
Tian Z, Zhou Y, Liu H. Extralobar pulmonary sequestration with absence of pericardium and atrial septal defect in a woman. J Cardiothorac Surg. 2019;14(1):113. https://doi.org/10.1186/s13019-019-0932-9.
Imperatori A, Rotolo N, Nardecchia E, Mariscalco G, Spagnoletti M, Dominioni L. Bronchogenic cyst associated with pericardial defect: case report and review of the literature. J Cardiothorac Surg. 2011;20(6):85. https://doi.org/10.1186/1749-8090-6-85.
Kamata T, Yoshida S, Iwata T, Nakatani Y, Yoshino I. Giant bronchogenic cyst with pericardial defect: a case report & literature review in Japan. J Thorac Dis. 2016;8:E684–8. https://doi.org/10.21037/jtd.2016.06.65.
Arinaga M, Tanaka K, Miura T, Chujo M, Hadama T, Uchida Y. A case of congenital partial pericardial defect and anomaly of phrenic nerve with cystic bronchiectasis. Jpn J Thorac Cardiovasc Surg. 1998;46:446–9 (in Japanese with English abstract).
Sakaguchi Y, Matsumoto K. Lobectomy for lung cancer with congenital pericardial defect. Ann Thorac Surg. 2019;108:e37–8.
Tsukada H, Ogawa I, Yamabe K, Endo S, Murayama F, Akaogi E, et al. A case of complete left pericardial defect found at operation for left lung cancer. Jpn J Chest Surg. 1989;3:115–9 (in Japanese with English abstract).
Saito R, Hotta F. Congenital pericardial defect associated with cardiac incarceration: case report. Am Heart J. 1980;100:866–70.
Ogawa J, Nagashima H, Suhara M, Yamauchi K. A case of left phrenic nerve paralysis caused by compression of the left auricle due to congenital partial left pericardial defect. J Jpn Respir Soci. 2009;47:200–4 (in Japanese with English abstract).
Gassner I, Judmaier W, Fink C, Lener M, Waldenberger F, Scharfetter H, et al. Diagnosis of congenital pericardial defects, including a pathognomic sign for dangerous apical ventricular herniation, on magnetic resonance imaging. Br Heart J. 1995;74:60–6.
Loebe M, Alexi-Meskhishvili V, Weng Y, Hausdorf G, Hetzer R. Use of polytetrafluoroethylene surgical membrane as a pericardial substitute in the correction of congenital heart defects. Tex Heart Inst J. 1993;20(3):213–7.
Kajiwara H, Hamada T, Ichikawa Y, Ishi M, Yamazaki I. Experience with expanded polytetrafluoroethylene (ePTFE Gore-Tex) surgical membrane for coronary artery grafting: does ePTFE surgical membrane predispose to postoperative mediastinitis? Artif Organs. 2004;28:840–5. https://doi.org/10.1111/j.1525-1594.2004.07298.x.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
None.
Author information
Authors and Affiliations
Contributions
YI participated in the surgery, conceived and conducted the study, did literature search. MI, SI, AY participated in the surgery, performed over all supervision of the manuscript and critical revision of the manuscript. NM and HU performed over all supervision of the manuscript and critical revision of the manuscript. SY and KK critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this report and its accompanying images.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Iijima, Y., Ishikawa, M., Iwai, S. et al. Congenital partial pericardial defect discovered incidentally during surgery for lung cancer: a case report and literature review. BMC Surg 21, 447 (2021). https://doi.org/10.1186/s12893-021-01453-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-021-01453-3