Abstract
Background
Serous cysto-adenoma (SCA) is a rare benign neoplasm of the pancreas. SCA can mimic other pancreatic lesions, such as neuroendocrine tumours. 68Gallium-DOTA-peptide Positron Emission Tomography (PET) is able to image in vivo the over-expression of the somatostatin receptors, playing an important role for the identification of neuroendocrine neoplasms.
Case presentation
We reported a case of 63-year-old man, with a solid lesion of 7 cm of diameter of the body–tail of the pancreas. Two fine-needle-aspirations (FNA) were inconclusive. A 68Ga-DOTA-peptide PET-CT revealed a pathological uptake of the pancreatic lesion. The diagnosis of a pancreatic neuroendocrine neoplasm was established and a laparoscopic distal splenopancreatectomy and cholecystectomy was performed. Final histopathological report revealed the presence of a micro-cystic SCA.
Conclusions
The current case firstly reports a pancreatic SCA showing increased radiopharmaceutical uptake at 68Ga-DOTA-peptide PET-CT images. This unexpected finding should be taken into account during the diagnostic algorithm of a pancreatic lesion, in order to minimize the risk of misdiagnosis and overtreatment of SCA.
Similar content being viewed by others
Background
Serous cysto-adenoma (SCA) is a rare benign neoplasm of the pancreas [1]. SCA can mimic other pancreatic lesions: about 10% of pancreatic neuroendocrine tumours (pNET) appears cystic on imaging and can be misdiagnosed with SCA [2]. 68Gallium-DOTA-peptide Positron Emission Tomography (PET) is able to image in vivo the over-expression of the somatostatin receptors, playing an important role for the identification of neuroendocrine neoplasms [3].
The following case-report firstly describes a pancreatic SCA showing uptake to 68-Gallium-DOTA-peptide. This case confirms the diagnostic challenge offered by pancreatic cystic neoplasms and encourages future investigations on the usefulness of 68Ga-DOTA-peptide in discriminating pancreatic SCA from pancreatic NET.
The following case report was written following the CARE Guidelines [4].
Case presentation
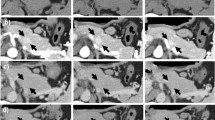
A 63-year-old man, without relevant past medical history, was referred to undergo an abdominal computed tomography scan (CT), due to a diffuse mild abdominal pain. The patient describes that the pain hadn’t any specific feature, not aggravating or alleviating from some specific factors, it appeared one time each week, it wasn’t invalidating and it disappeared without adoption of any medications. No fever, vomiting, diarrhea or weight loss were present. CT images showed a lesion sized 7 cm of diameter of the body–tail of the pancreas (Fig. 1a). An Endoscopic Ultrasound (EUS) showed a vascularized hypoechoic lesion with exophytic growth in the body of the pancreas. A fine needle aspiration (FNA) was performed, showing the presence of epithelial cells, without atypia (category II according the Papanicolaou Society of Cytopathology Guidelines) [5]. One month after, a new EUS with FNA was repeated, but the results was inconclusive (category I according the Papanicolaou Society of Cytopathology Guidelines) [5]. In both endoscopic examinations, a dosage of intra-cystic glucose, CA 19.9 and CEA was not performed. A magnetic resonance (MRI) was performed, confirming the expansive solid lesion, with multiple septa inside, strictly adherent to the jejunum (Fig. 1b). Laboratory tests were normal, as well as Ca 19.9 value. After these investigations, we had two diagnostic theories: (1) a pancreatic SCA, considering the presence multiple septa delimitating small cystic spaces, even if the typical central scar was missing; (2) a pancreatic NET, due to the presence of a solid lesion highly vascularized. In order to discriminate these two disease, 2 months after, a 68Ga-DOTA_peptide PET-CT was performed, revealing a pathological uptake of the pancreatic lesion (SUV max 9.6; mean 7.1) (Fig. 2). A diagnosis of pancreatic neuroendocrine tumour was established, with consequently indication to surgery [6]. A distal splenopancreatectomy and cholecystectomy with laparoscopic approach was performed: intraoperatively, we confirmed the presence of a large solid mass, hyper-vascularized, strictly adherent to mesocolon. The lesion was extremely attached to splenic vessel and near to the spleen: for these reasons, was not possible to performed a distal pancreatectomy spleen preserving. Post-operative course was uneventful and the patient was discharged on post-operative day (POD) [7]. Final histopathological report was diagnostic for microcystic CSA: the cysts were lined by a single layer of uniform, cuboidal, epithelial cells with clear cytoplasm (due to abundant glycogen, demonstrated at histochemical analysis by the positivity for Periodic Acid-Schiff (PAS) and negativity for PAS-diastase) and central oval hyperchromatic nuclei with inconspicuous nucleoli; the central scar and radiating septa was composed of hyalinised collagen (Fig. 3a). Immunohistochemical analysis (IHC) revealed for the cystic lesion negativity for chromogranin A, synaptophysin and vimentin (Fig. 3b–d).
Histology of the specimen: a the cysts were lined by a single layer of uniform, cuboidal, epithelial cells with clear cytoplasm (due to abundant glycogen) and central oval hyperchromatic nuclei with inconspicuous nucleoli; the central scar and radiating septa was composed of hyalinised collagen. Hematoxylin and Eosin staining (magnification 10×); b–d Immuno-histochemical analysis for Synaptophisin (b), Vimentin (c) and Chromogranin (d), in which, only Langerhans’ islet (Red arrow), and not the cystic lesion (Blue arrows), showed positivity for chromogranin A
Discussion and conclusions
SCAs are rare, benign neoplasms of pancreas [1]. The mean age of presentation is 60 years and women are more frequently affected. The majority of SCAs are asymptomatic and incidentally discovered. Abdominal pain, nausea and palpable mass are the most common presenting symptoms [7]. International guidelines stated that SCA is a benign entity [8]. For this reason, surgical treatment of these lesions is not recommended, except in case of patients with symptoms related to the compression of adjacent organs (bile duct, stomach, duodenum, portal vein) [8]. However, in literature large series of resected pancreatic cystic lesions comprise patients with a final diagnosis of SCA, including 40–60% of asymptomatic SCA [2, 9, 10]. The reason of this not negligible rate of “useless” resection for asymptomatic SCA is that in some cases can be difficult to differentiate this cystic lesion from mucinous or neuroendocrine one [11, 12]. CT-scan, MRI and Endoscopic Ultrasound (EUS) are useful diagnostic tools, that should improve the accuracy of diagnosis, but they can’t delete the risk of misdiagnosis [13]. In fact, in the current case, the FNA procedure was not useful for diagnostic confirmation. The Fine Needle Biopsy (FNB) might be use as alternative investigation, in order to catch the core of pathology; nevertheless, FNB could be used also to perform cytological specimens (through tissue apposition).
One of the most difficult differential diagnoses of SCA is represented by neuroendocrine pancreatic neoplasms. Typically, neuroendocrine lesions, especially the well differentiated neuroendocrine tumors, appear as hyper-vascular masses on abdominal CT scan and 11% of them appear cystic. The peripheral rim hyper-enhancement is a typical feature described in 85% of cystic pancreatic neuroendocrine lesions, which is usually not present in SCA [11]. The 68Ga-DOTA_peptide PET-CT is now becoming the standard molecular imaging technique recommended in most current guidelines for diagnosis and staging of neuroendocrine tumours. There is a strong established application of the somatostatin receptor PET imaging in pancreatic neuroendocrine lesions, showing a sensitivity and predictive positive value up to 100% [2]; on the other hand, false positives have been reported [14]. This imaging finding mislead the diagnosis, suggesting a of neuroendocrine pancreatic neoplasm and implied a wrong indication to surgical treatment. The patient was asymptomatic/few symptomatic and he could have been theoretically managed with periodical follow-up. Mixed serous-neuroendocrine neoplasm (MSNN) of the pancreas is a distinct clinicopathologic entity, defined as a tumor containing two components with different pathologies [15]. Several cases of MSNN have been reported [16]. Our case was not a MSNN, because at pathological examination there wasn’t any evidence of neuroendocrine features. To our knowledge, in literature this case firstly reported a pancreatic SCA showing pathological tracer uptake at 68Ga-DOTA-peptide. For this reasons, we are not allowed to extend this evidence to all other pancreatic SCA. On the other hand, this finding has to be taken to into account, in order to minimize the risk of misdiagnosis in the differential diagnosis between SCA and neuroendocrine pancreatic lesions and to avoid an overtreatment of the patient. We strongly encourage future studies evaluating the relationship between pancreatic SCAs and Ga-60-DOTA-peptide.
Availability of data and materials
Yes.
References
Colonna J, Plaza JA, Frankel WL, et al. Serous cystadenoma of the pancreas: clinical and pathological features in 33 patients. Pancreatology. 2008;8:135–41.
Chu LC, Singhi AD, Haroun RR, Hruban RH, Fishman EK. The many faces of pancreatic serous cystadenoma: radiologic and pathologic correlation. Diagn Interv Imaging. 2017;98:191–202.
Kumar R, Sharma P, Garg P, Karunanithi S, Naswa N, Sharma R, Thulkar S, Lata S, Malhotra A. Role of (68)Ga-DOTATOC PET-CT in the diagnosis and staging of pancreatic neuroendocrine tumours. Eur Radiol. 2011;21:2408–16.
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley DS, CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med. 2013;2(5):38–43.
Pitman M, Centno B, Alis S, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: the Papanicolau Society of Cytopathology Guidelines. Cytojournal. 2014;11:3.
Foulfoin M, Graillot E, Adham M, Rousset P, Forestier J, Hervieu V, Robinson P, Scoazec JY, Lombard-Bohas C, Walter T. Treatment of metastatic pancreatic neuroendocrine tumors: relevance of ENETS 2016 guidelines. Endocr Relat Cancer. 2017;24(2):71–81.
Horaguchi J, Fujita N, Kobayashi G, et al. Serous cystadenoma of the pancreas associated with obstructive jaundice. J Gastroenterol. 2003;38:501–6.
European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804.
Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, et al. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006;192:148–54.
Galanis C, Zamani A, Cameron JL, Campbell KA, Lillemoe KD, Caparrelli D, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820–6.
Sahani DV, Kambadakone A, Macari M, et al. Diagnosis and management of cystic pancreatic lesions. Am J Roentgenol. 2013;200:343–54.
Shah AA, Sainani NI, Kambadakone AR, et al. Predictive value of multi-detector computed tomography for accurate diagnosis of serous cystadenoma: radiologic-pathologic correlation. World J Gastroenterol. 2009;15:2739–47.
Hwang H, Yu SJ, Cho ES, Kim JH, Chung JJ. Serous cystic neoplasms of the pancreas: endoscopic ultrasonographic versus computed tomography and magnetic resonance imaging features of surgically removed masses. Ultrasound Q. 2018;34:122–7.
Skoura E, Michopoulou S, Mohmaduvesh M, et al. The impact of 68Ga-DOTATATE PET/CT imaging on management of patients with neuroendocrine tumors: experience from a national referral center in the United Kingdom. J Nucl Med. 2016;57:34–40.
Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumors of the digestive system. Lyon: World Health Organization; 2010.
Li Y, Dai M, Chang X, et al. Mixed serous neuroendocrine neoplasm of the pancreas: case report and literature review. Medicine. 2016;95:e4205–e4205.
Acknowledgements
No acknowledgements are needed.
Funding
No funding are needed.
Author information
Authors and Affiliations
Contributions
GN: conceived and designed the analysis; collected the data, wrote the paper; NF: critical revision of the paper. SG: collected the data, wrote the paper. PG: collected the data; contributed data; critical revision of the paper. GC, SC: contributed data. GP, AZ: contributed data; critical revision of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval was required.
Consent for publication
Written informed consent was obtained by study participant.
Competing interests
None of the authors have conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nappo, G., Funel, N., Giudici, S. et al. Pancreatic serous cystoadenoma (CSA) showing increased tracer uptake at 68-GaDOTA-peptide Positron Emission Tomography (68Ga-DOTA-peptide PET-CT): a case report. BMC Surg 20, 331 (2020). https://doi.org/10.1186/s12893-020-01004-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-020-01004-2