Abstract
Background
With life expectancy on the rise, there has been an increase in patients with concomitant degenerative hip and spine pathology, defined as hip-spine syndrome (HSS). Patients affected by HSS may require both total hip arthroplasty (THA) and lumbar spinal fusion (LSF), although there is a paucity of data regarding how the sequential timing of these procedures may influence clinical outcomes. This study aims to compare complications and spinopelvic parameters in patients with HSS who underwent either LSF first or THA first.
Methods
A systematic search of PubMed and Scopus was conducted for randomized and nonrandomized studies investigating complications and spinopelvic parameters in patients with HSS who had undergone THA and LSF. The Methodological Index for Non-Randomized Studies (MINORS) tool was utilized to assess the risk of bias in included studies. Relevant outcomes were pooled for meta-analysis.
Results
Eleven articles were included in this study. There was a significantly higher THA dislocation rate in patients who had undergone LSF first compared to those who had THA first (OR: 3.17, 95% CI 1.23–8.15, P = 0.02). No significant difference was found in terms of THA aseptic loosening (OR: 0.86; 95% CI 0.32–2.32, p = 0.77) and revision rate (OR: 1.18, 95% CI: 0.53–2.62) between these two groups. Individuals who received THA only showed a significantly lower risk of hip dislocation (OR: 0.14, 95% CI: 0.08–0.25, P < 0.00001) and THA revision (OR: 0.22, 95% CI: 0.14–0.36, P < 0.00001) compared to patients with a previous LSF.
Conclusions
In HSS patients who underwent both LSF and THA, those who received LSF first displayed an increased risk of hip dislocation after subsequent THA. Additionally, the relative risks of dislocation and revision rate appeared significantly lower in patients who had undergone THA only when compared to THA patients with a history of previous LSF. Due to the impact of LSF on spinopelvic biomechanics, caution must be exercised when performing THA in individuals with instrumented spines.
PROSPERO ID
CRD42023412447.
Level of evidence
LL.
Similar content being viewed by others
Background
Degenerative hip and spine disorders are becoming more prevalent in the aging population, especially with the global increase of life expectancy. Among adults above the age of 60, 19–47% are estimated to have spinal stenosis [1]. Furthermore, hip osteoarthritis (OA) has been estimated to nearly affect 7.95% of adults in North America, with prevalence increasing with age [2, 3]. Oftentimes, elderly adults may present with radiographic evidence of both hip and spine degenerative pathologies, and the overlap in symptomatology between the two can portend a complex clinical picture. This concomitant presence of degenerative hip and spine pathology has been termed hip-spine syndrome (HSS) [4].
In patients with severe hip OA and advanced degenerative changes of the spine, it can be difficult to ascertain which condition is most symptomatic and, thus, which pathology to address first. Severe degenerative LSS is typically managed with decompressive surgery such as laminectomy or foraminotomy [5, 6]. However, lumbar spinal fusion (LSF) may be required in addition to bony decompression, especially in the case of concomitant spondylolisthesis, degenerative scoliosis, or following extensive removal of stabilizing structures to avoid iatrogenic instability. The standard of care for symptomatic end-stage hip OA is total hip arthroplasty (THA), which has been shown to be highly successful and cost-effective [7].
Although both THA and LSF are effective treatments for symptomatic hip and spine pathologies, less is known about outcomes in patients who require both interventions. Previous studies have indicated that a history of LSF can lead to increased rates of hip dislocation and revision surgery after subsequent THA due to the interrelation of spinopelvic biomechanics [8]. Careful consideration of the spinopelvic balance during preoperative planning is advised in patients with a previous LSF due to the strict relationships between spinopelvic parameters and clinical outcomes and complications following THA [9]. However, there is a paucity of data regarding the clinical outcomes and spinopelvic balance of patients with HSS who have undergone both THA and LSF, and whether the sequence of surgery affects these variables. The primary objective of this systematic review and meta-analysis was to compare postoperative complications (e.g., THA dislocation, revision, aseptic loosening, infection) in patients affected by HSS who underwent both LSF and THA, taking into consideration surgical timing (LSF first vs. THA first). Our secondary objective was to assess the effect of surgery sequencing on radiographic indicators of spinopelvic balance (e.g., sacral slope, pelvic tilt, pelvic incidence, lumbar lordosis, etc.).
Materials and methods
This review was conducted in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The review protocol has been approved by the International Prospective Register of Systematic Reviews (PROSPERO) under the ID CRD42023412447.
Literature search
A systematic search was performed on May 9, 2024 using the PubMed and Scopus databases for literature published before May 2024. According to the PICOS framework, we searched for studies including adult individuals diagnosed with HSS (P) who had undergone LSF (I) and THA (C), reported which procedure had been completed first, and investigated postoperative complications and spinopelvic parameters (O). Randomized controlled trials (RCTs), prospective and retrospective observational cohort studies and case series with ≥ 10 patients per group were included (S). Non-English language studies, case series with < 10 patients, case reports, reviews, database studies, editorials, book chapters, and studies that did not delineate the order in which the surgical procedures were performed, were excluded. The complete search strategy is available as a Supplementary File.
Study selection
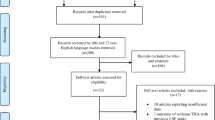
The initial search of the articles was independently conducted by two reviewers (LA and AH). The following research order was used: titles and abstract were screened first, then full texts of papers not excluded based on abstract nor title were analyzed. Conflicts regarding the inclusion of studies were mutually resolved after a thorough discussion between the two screening authors. The article screening workflow is reported in a PRISMA flow diagram (Fig. 1).
Data extraction
General study characteristics extracted included: authors, country, sample size, mean age, mean follow-up, study design, level of evidence (LOE), year of publication, demographic characteristics, and data regarding both the interventions (type of LSF technique, number of levels involved, extension to the pelvis and/or sacrum) and comparator (type of THA approach, type of implant utilized). Outcomes collected included spinopelvic radiographic measurements, namely sacral slope (SS), lumbar lordosis (LL), pelvic tilt (PT), pelvic incidence (PI), PI-LL mismatch, femoral offset (FO), femoral angle (FA), acetabular inclination (AI), acetabular anteversion (AA), acetabular anteinclination angle (AAA), and sagittal vertical axis (SVA). Moreover, postoperative complications were reported.
Risk of bias
The Methodological Index for Non-Randomized Studies (MINORS) tool for non-randomized clinical trials [11] was used to assess the risk of bias in included studies. Papers were independently rated by two reviewers (LA and AH) and verified by a third reviewer (FR).
Statistical analysis
Meta-analysis was performed using odds ratios (OR) with 95% confidence intervals (Cl) to describe categorical variables. The level of significance was set at 0.05. Heterogeneity among comparisons was calculated by the I2 statistics and classified as “low” (I2 ≤ 25%), “moderate” (I2 = 26–74%), or “high” (I2 ≥ 75%). Pooled estimates were calculated by the Mantel-Haenszel method for dislocation, aseptic loosening, and revision rates. Considering the high variability among different surgical approaches and implants utilized to perform THA in included studies, no meta-analysis of spinopelvic parameters was performed. Random effect models were employed when heterogeneity was statistically significant; otherwise, a fixed effect model was applied. Due to the low number of studies per single outcome, publication bias was not evaluated. Formal analysis was performed with Review Manager (v. 5.4, Cochrane Collaboration, UK).
Results
Study selection
The initial search from the databases yielded 2,296 articles. After duplicates were removed, 2,239 unique articles remained. 2,218 articles were excluded following title and abstract screening. Six additional studies were found through manual citation searching. Then, 27 full-text articles were screened and 16 were excluded due to various reasons (noncomparative studies = 4, inappropriate outcomes = 5, inappropriate populations = 7). Eventually, 11 articles met the eligibility criteria and were included (Fig. 1).
Study characteristics
Included articles consisted of ten retrospective cohort studies [12,13,14,15,16,17,18,19,20,21,22] and one case-control study [23] published between 2016 [12] and 2024 [22] from the USA [14, 16, 18,19,20, 22], Italy [13], Japan [15], UK [17], France [23], and China [21]. Collectively, a total of 4,508 patients were assessed: 2,129 patients underwent LSF first followed by THA, 747 underwent THA first followed by LSF, and 1,632 underwent THA only, with a mean age of 65.7, 63.9, and 63.4 years, respectively. Follow-up ranged from a minimum of 6 months [15] to 5.2 years [13] (Table 1). The surgical characteristics of included patients are summarized in Table 2. According to the MINORS tool, the average score of included studies was 15.7/24, which is indicative of a substantial risk of bias (Table 1).
Clinical outcomes
Seven studies [13,14,15,16,17, 21, 22] compared patients who underwent both THA and LSF with specific mention of which procedure was performed first. Across these studies, there were 1,032 patients who underwent LSF first, and 760 patients who underwent THA first. There was a significantly higher hip dislocation rate in patients who had undergone THA after LSF compared to THA before LSF (5 studies, LSF first, n = 948; THA first, n = 674; OR: 3.17, 95% CI 1.23–8.15, P = 0.02, Fig. 2A). The sequence of surgery had no significant effect on THA aseptic loosening (3 studies, LSF first, n = 470; THA first, n = 391; OR: 0.86; 95% CI 0.32–2.32, P = 0.77, Fig. 2B) and revision rate (2 studies, LSF first, n = 397; THA first, n = 329; OR: 1.18, 95% CI: 0.53–2.62, Fig. 2C). Khan et al. [22] showed no statistically significant difference between patients undergoing LSF or THA first in terms of 30-day and 90-day readmission rates. Similarly, Di Martino et al. [13] reported that the sequence of surgery did not significantly contribute to hip implant breakage (LSF first: 0%, THA first: 0.7%), polyethylene insert wear (LSF first: 0%, THA first: 1%), and hip instability (LSF first: 0.5%, THA first: 0%). The authors also showed a significantly higher mechanical complication rate (e.g., dislocation, hip instability, etc.) during the first two years following THA in patients undergoing THA + LSF vs. THA alone, as well as in patients undergoing LSF first vs. THA first. Likewise, Perfetti and colleagues [19] demonstrated that medium times to dislocation and revision surgeries were 170.8 and 139.3% shortened in patients with a prior LSF compared to patients who received THA alone.
Four studies [18,19,20, 23] compared 1,097 patients who underwent LSF first followed by THA with 1,619 patients who received THA only. Individuals who received THA only had a significantly lower risk of hip dislocation (3 studies, THA only, n = 1571; LSF + THA, n = 1067; OR: 0.14, 95% CI: 0.08–0.25, P < 0.00001, Fig. 3A) and revision rate (2 studies, THA only, n = 1013; LSF + THA, n = 978; OR: 0.22, 95% CI: 0.14–0.36, P < 0.00001, Fig. 3B) compared to patients with a previous LSF. Barry and colleagues [20] reported that during the first 90 postoperative days patients who underwent LSF first presented a significantly higher rate of nonsurgical complications (i.e., superficial wound infection, pneumonia, intensive care unit transfer, delirium, conservatively treated dislocation, fall, C. difficile infection) compared to patients undergoing primary THA (20 vs. 5.7%, P = 0.039).
Spinopelvic parameters
Five studies [14, 15, 17, 21, 23] investigated spinopelvic parameters (Table 3). In patients who received both THA and LSF, Furuhashi et al. [15] reported that a higher PT and a lower AA were significantly associated with increased postoperative dislocations. Grammatopoulos et al. [17] did not show any significant difference between patients who underwent LSF first and THA first in terms of SS, AI, and AA. Zhang et al. [21] did not find any substantial differences concerning LL, PT, SS, and SVA, although AA was significantly lower in subjects who received LSF first. Goyal and coauthors [14] evaluated the differences among spinopelvic parameters between the same two groups, although comparing values subgrouping patients based on the surgical approach to the hip (direct anterior vs. direct lateral). No significant differences between the two techniques in patients who underwent LSF first or THA first were reported in terms of LL, SS, PT, PI, FA, AI, and AAA. The PI-LL mismatch was significantly lower in patients who underwent THA with a direct lateral approach after LSF compared to a direct anterior approach, while there was no difference in PI-LL mismatch between the anterior and lateral hip approaches in patients who underwent THA before LSF. Similarly, in the same group, FO was significantly lower with the direct anterior approach than with the direct lateral approach, whereas no difference was found in patients who received THA first with both techniques. In another study, Lazennec et al. [23] reported that patients who underwent LSF first did not show relevant differences in terms of standing AI compared to subjects who received THA only. However, mean standing SS, sitting SS, sitting AI, standing AA, and sitting AA significantly differed between the two groups. Interestingly, the mean SS change from standing to sitting position was significantly lower in patients with a previous LSF, as well as the mean AI change and AA change. The authors found that, for each additional fused level, the mean SS change decreased by 1.6°, the mean AI change decreased by 0.8°, and the mean AA change decreased by 0.9°. Furthermore, decreases in these indices were even higher when comparing LSF patients who received a lumbar fusion with individuals who underwent a thoracolumbar or lumbosacral fusion.
Discussion
Considering the increased life expectancy and higher prevalence of age-related musculoskeletal diseases, the burden of hip and spine degenerative disorders is presumed to promptly rise in the near future [1, 2]. These two conditions often overlap in the complex and multifaceted HSS, which frequently requires surgical care [4]. According to previous evidence, the treatment of symptomatic HSS is usually based on the predominant complaint, although it is not uncommon to perform both LSF and THA in the same patient [24]. Due to the complex biomechanical relationships between the hip and the spine, decreased sagittal motion after LSF has been shown to accommodate less for PT changes and consequently affect femoral impingement, which may ultimately cause hip dislocation following subsequent THA [25].
In this study, our meta-analysis showed that patients who have undergone LSF prior to THA present a 3.2-fold higher risk of dislocation compared to subjects who received LSF after THA. Conversely, there was no statistically significant difference in the risk of aseptic loosening and revision surgery between these two groups. However, when comparing patients who have undergone THA only versus patients who have received LSF first followed by THA, the latter showed an 86% higher risk of dislocation and a 78% higher risk of revision. This is in line with the outcomes of a previous large database study demonstrating that patients with prior LSF undergoing THA had a 106% increased risk of dislocation compared to those with LSF done five years after THA [26]. Conversely, another database study recently showed no differences in dislocation emerged between patients undergoing LSF in the year prior or in the year after THA [27]. Previous studies have also reported an increased rate of periprosthetic joint infections in patients who underwent LSF first. This has been imputed to a combination of higher opioid consumption, increased risk of falls, delirium, and pneumonia [17].
Physiologically, when moving from standing to sitting, the pelvis tilts posteriorly, the SS decreases, and AA increases, therefore providing anterior clearance to allow the proximal femur to flex more. In the fused spine, the posterior PT is abolished or even paradoxically inverted, thus posing the risk of femoral impingement and subsequent posterior dislocation [28]. In addition, the reduced posterior PT when sitting is frequently compensated by increased hip flexion, which further contributes to the higher risk of dislocation [29]. Collectively, these mechanisms may explain the increased rate of mechanical complications in patients undergoing THA after being treated with a previous LSF. Indeed, previous studies have demonstrated that THA patients who had a prior LSF reported a dislocation rate ranging from 3.0% at one year to 7.5% at two years compared to 0.4 to 2.1% dislocation rate in controls who have undergone THA only. Moreover, when stratifying cohorts based on the number of fused segments, the occurrence of complications significantly increased with the number of fused levels [30]. While spinopelvic parameters were collected as part of this review, there was not enough standardized data across the studies to perform meta-analysis. This may represent an area for future research, given that spinopelvic measures are essential in surgical planning and post-operative assessment of stability and dislocation risk. York et al. [18] found that among patients who have undergone both THA and LSF, dislocators had a significantly lower PI and SS compared to non-dislocators [24]. Notably, all included studies reported average PI values exceeding 50.0°. A high PI has been previously associated to an elevated risk of accelerated disc degeneration and hip OA, due to the increased mechanical forces transmitted to the lumbar spine and femoral head, respectively. Therefore, it seems reasonable that patients with higher PI values may be characterized by a higher risk of HSS [31]. Altogether, these findings do provide helpful information for orthopaedic spine surgeons when planning which intervention to perform first in a patient who may need both THA and LSF.
Considering the notable burden of complications in individuals with THA and a prior LSF, a systematic approach should be adopted for high-risk patients when planning THA to avoid dislocation. First, standing and sitting lateral spinopelvic radiographs should be obtained to measure spinopelvic parameters and their change with posture. A spine with an SS change < 20.0° from standing to sitting should be considered stiff. In that case, the cup should be anteverted more to accommodate femoral flexion when sitting, ideally, 30.0° planned to the functional pelvic plane or the higher end of the traditional safe zones (15.0–20.0°) [28, 32]. Interestingly, a recent study showed that 80% of dislocators with spinal deformity had a cup anteversion value within the range defined by the traditional Lewinnek safe zone [33]. This further delineates the importance of accurately planning cup anteversion based on each individual type and severity of spinal stiffness. On the other hand, when treating hip OA first in a patient affected by moderately symptomatic LSS, it is difficult to anticipate whether LSS will be necessary in the future and plan any possible technical adjustments accordingly. In this setting, the increased risk of posterior dislocation may be reduced by the selection of an anterior approach with less damage to the posterior capsule and external rotators. Some authors have reported the use of a range of motion simulation test using a three-dimensional software to assess the implant biomechanics and properly adjust the cup orientation preoperatively [15]. Furthermore, the use of high-resistant components (e.g., vitamin E-stabilized ultrahigh molecular weight polyethylene), materials with a favorable tribological profile, large-diameter femoral heads, and dual mobility constructs may be indicated to reduce the risk of dislocation and mechanical complications [34]. Whereas the priority between LSF and THA is dictated by the clinical picture, previous studies have demonstrated that in case both surgical procedures are needed, waiting at least one year between LSF and THA is advisable. According to Parilla et al. [16], further compensatory PT alterations are less common at one year following LSF, thus rendering all the needed adjustments based on PT change more reliable after such time point.
While this review highlights the complications and spinopelvic parameters in patients undergoing both THA and LSF, there are a paucity of data regarding patient-reported outcomes in patients who undergo both procedures. Eneqvist et al. [35] found that patients who underwent lumbar surgery prior to THA generally had more pain, worse health-related quality of life scores and were less satisfied with their THA outcome one year postoperatively. Similarly, Grammatopoulos et al. [17] demonstrated that patients with a prior LSF showed significantly lower Oxford Hip Score values compared to patients undergoing LSF after THA. However, additional research is needed to determine if there are significant differences in outcome measures with regard to timing of performing LSF first or THA first in patients who must undergo both procedures.
This study has some limitations. All included studies were nonrandomized retrospective cohort studies including one case-control study, thus being inherently characterized by a low level of evidence. Randomized, prospective, high-quality clinical trials are therefore needed to further confirm our data. In addition, the average MINORS score was indicative of a substantial risk of bias, which may have an impact on the reliability of the data that were reported. Some of the analyzed outcomes included a small number of studies and/or were reported at different time points, thus further limiting the generalizability of our findings and possibly introducing confounding. Furthermore, we did not include studies on patients with HSS who were treated with lumbar decompression only, such as laminectomy or laminotomy. However, considering the smaller effect of these procedures on spinopelvic balance, uninstrumented spinal decompression may affect the risk of hip-related complications to a lesser extent. None of the included studies recruited patients who underwent LSF only, although the outcomes of interest in our study were mainly related to THA complications, which would not be applicable in that cohort. In addition, due to inconsistent reporting among included studies, our analysis did not take into account the number of fused levels and the involvement of the lumbosacral junction. Indeed, longer fusions may reasonably further increase the risk of hip mechanical complications. Nonetheless, further investigation into outcomes of patients with HSS who undergo THA and decompressive surgery without instrumentation is warranted.
Conclusions
In patients who undergo both LSF and THA, patients who underwent LSF first are at increased risk of hip dislocation after subsequent THA. In the setting of a previously fused spine, careful THA planning is paramount to prevent further complications and the risk of revision surgery.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AA:
-
Acetabular anteversion
- AAA:
-
Acetabular anteinclination angle
- AI:
-
Acetabular inclination
- CI:
-
Confidence interval
- FA:
-
Femoral angle
- FO:
-
Femoral offset
- HSS:
-
Hip-spine syndrome
- LOE:
-
Lumbar lordosis
- LSF:
-
Lumbar spinal fusion
- LSS:
-
Lumbar spinal stenosis
- MINORS:
-
Methodological Index for Non-Randomized Studies
- OA:
-
Osteoarthritis
- OR:
-
Odds ratio
- PI:
-
Pelvic incidence
- PRISMA:
-
Preferred Reporting Items of Systematic Reviews and Meta-Analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- PT:
-
Pelvic tilt
- RCT:
-
Randomized controlled trial
- SS:
-
Sacral slope
- SVA:
-
Sagittal vertical axis
- THA:
-
Total hip arthroplasty
References
Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, Hunter DJ. Spinal stenosis prevalence and association with symptoms: the Framingham study. Spine J. 2009;9(7):545–50.
Fan Z, Yan L, Liu H, Li X, Fan K, Liu Q, Li JJ, Wang B. The prevalence of hip osteoarthritis: a systematic review and meta-analysis. Arthritis Res Therapy. 2023;25(1).
Cannata F, Laudisio A, Ambrosio L, Vadalà G, Russo F, Zampogna B, Napoli N, Papalia R. The association of body mass index with surgical time is mediated by comorbidity in patients undergoing total hip arthroplasty. J Clin Med. 2021;10(23).
Buckland AJ, Miyamoto R, Patel RD, Slover J, Razi AE. Differentiating hip pathology from lumbar spine pathology: key points of evaluation and management. J Am Acad Orthop Surg. 2017;25(2):e23–34.
Ma X-l, Zhao X-w, Ma J-x, Li F, Wang Y, Lu B. Effectiveness of surgery versus conservative treatment for lumbar spinal stenosis: a system review and meta-analysis of randomized controlled trials. Int J Surg. 2017;44:329–38.
Mallio CA, Vadalà G, Russo F, Bernetti C, Ambrosio L, Zobel BB, Quattrocchi CC, Papalia R, Denaro V. Novel magnetic resonance imaging tools for the diagnosis of degenerative disc disease: a narrative review. Diagnostics. 2022;12(2).
Murphy NJ, Eyles JP, Hunter DJ. Hip osteoarthritis: etiopathogenesis and implications for management. Adv Therapy. 2016;33(11):1921–46.
Onggo JR, Nambiar M, Onggo JD, Phan K, Ambikaipalan A, Babazadeh S, Hau R. Clinical outcomes and complication profile of total hip arthroplasty after lumbar spine fusion: a meta-analysis and systematic review. Eur Spine J. 2019;29(2):282–94.
Louette S, Wignall A, Pandit H. Spinopelvic relationship and its impact on total hip arthroplasty. Arthroplasty Today. 2022;17:87–93.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–6.
Sing DC, Barry JJ, Aguilar TU, Theologis AA, Patterson JT, Tay BK, Vail TP, Hansen EN. Prior lumbar spinal arthrodesis increases risk of prosthetic-related complication in total hip arthroplasty. J Arthroplast. 2016;31(9):227–e232221.
Di Martino A, Bordini B, Ancarani C, Viceconti M, Faldini C. Does total hip arthroplasty have a higher risk of failure in patients who undergo lumbar spinal fusion? Bone Joint J. 2021;103–B(3):486–91.
Goyal DKC, Divi SN, Vaccaro AR, Hozack WJ. Stability in direct lateral vs direct anterior total hip arthroplasty in the context of lumbar spinal fusion. J Am Acad Orthop Surg. 2022;30(7):e628–39.
Furuhashi H, Yamato Y, Hoshino H, Shimizu Y, Hasegawa T, Yoshida G, Banno T, Arima H, Oe S, Ushirozako H, et al. Dislocation rate and its risk factors in total hip arthroplasty with concurrent extensive spinal corrective fusion with pelvic fixation for adult spinal deformity. Eur J Orthop Surg Traumatol. 2020;31(2):283–90.
Parilla FW, Shah RR, Gordon AC, Mardjetko SM, Cipparrone NE, Goldstein WM, Goldstein JM. Does it matter: total hip arthroplasty or lumbar spinal fusion first? Preoperative sagittal spinopelvic measurements guide patient-specific surgical strategies in patients requiring both. J Arthroplast. 2019;34(11):2652–62.
Grammatopoulos G, Dhaliwal K, Pradhan R, Parker SJM, Lynch K, Marshall R, Andrade AJ. Does lumbar arthrodesis compromise outcome of total hip arthroplasty? HIP Int. 2018;29(5):496–503.
York PJ, McGee AW, Dean CS, Hellwinkel JE, Kleck CJ, Dayton MR, Hogan CA. The relationship of pelvic incidence to post-operative total hip arthroplasty dislocation in patients with lumbar fusion. Int Orthop. 2018;42(10):2301–6.
Perfetti DC, Schwarzkopf R, Buckland AJ, Paulino CB, Vigdorchik JM. Prosthetic dislocation and revision after primary total hip arthroplasty in lumbar fusion patients: a propensity score matched-pair analysis. J Arthroplast. 2017;32(5):1635–e16401631.
Barry JJ, Sing DC, Vail TP, Hansen EN. Early outcomes of primary total hip arthroplasty after prior lumbar spinal fusion. J Arthroplast. 2017;32(2):470–4.
Zhang H, Yu H, Zhang M, Huang Z, Xiang L, Liu X, Wang Z. Selection of spinal surgery and hip replacement sequence in patients with both degenerative scoliosis and hip disease. J Int Med Res. 2020;48(12).
Khan IA, Cozzarelli NF, Sutton R, Ciesielka K-A, Arshi A, Fillingham YA. Patients requiring both total hip arthroplasty and lumbar spinal fusion have lower hip functional outcome scores: a matched case-control study. J Arthroplast. 2024;39(5):1291–7.
Lazennec JY, Clark IC, Folinais D, Tahar IN, Pour AE. What is the impact of a spinal fusion on acetabular implant orientation in functional standing and sitting positions? J Arthroplast. 2017;32(10):3184–90.
Devin CJ, McCullough KA, Morris BJ, Yates AJ, Kang JD. Hip-spine syndrome. J Am Acad Orthop Surg. 2012;20(7):434–42.
Lee SH, Lim CW, Choi KY, Jo S. Effect of spine-pelvis relationship in total hip arthroplasty. Hip Pelvis. 2019;31(1).
Malkani AL, Himschoot KJ, Ong KL, Lau EC, Baykal D, Dimar JR, Glassman SD, Berry DJ. Does timing of primary total hip arthroplasty prior to or after lumbar spine fusion have an effect on dislocation and revision rates? J Arthroplast. 2019;34(5):907–11.
Welling S, Smith S, Yoo J, Philipp T, Mildren M, Kagan R. Is timing of total hip arthroplasty and lumbar spine fusion associated with risk of hip dislocation? Arthroplasty Today. 2023;23.
Eftekhary N, Shimmin A, Lazennec JY, Buckland A, Schwarzkopf R, Dorr LD, Mayman D, Padgett D, Vigdorchik J. A systematic approach to the hip-spine relationship and its applications to total hip arthroplasty. Bone Joint J. 2019;101–B(7):808–16.
Esposito CI, Miller TT, Kim HJ, Barlow BT, Wright TM, Padgett DE, Jerabek SA, Mayman DJ. Does degenerative lumbar spine disease influence femoroacetabular flexion in patients undergoing total hip arthroplasty? Clin Orthop Relat Res. 2016;474(8):1788–97.
Sultan AA, Khlopas A, Piuzzi NS, Chughtai M, Sodhi N, Mont MA. The impact of spino-pelvic alignment on total hip arthroplasty outcomes: a critical analysis of current evidence. J Arthroplast. 2018;33(5):1606–16.
Morimoto T, Kobayashi T, Tsukamoto M, Hirata H, Yoshihara T, Toda Y, Mawatari M. Hip–spine syndrome: a focus on the pelvic incidence in hip disorders. J Clin Med. 2023;12(5).
Phan D, Bederman SS, Schwarzkopf R. The influence of sagittal spinal deformity on anteversion of the acetabular component in total hip arthroplasty. Bone Joint J. 2015;97–B(8):1017–23.
DelSole EM, Vigdorchik JM, Schwarzkopf R, Errico TJ, Buckland AJ. Total hip arthroplasty in the spinal deformity population: does degree of sagittal deformity affect rates of safe zone placement, instability, or revision? J Arthroplast. 2017;32(6):1910–7.
Boyer B, Philippot R, Geringer J, Farizon F. Primary total hip arthroplasty with dual mobility socket to prevent dislocation: a 22-year follow-up of 240 hips. Int Orthop. 2011;36(3):511–8.
Eneqvist T, Nemes S, Brisby H, Fritzell P, Garellick G, Rolfson O. Lumbar surgery prior to total hip arthroplasty is associated with worse patient-reported outcomes. Bone Joint J. 2017;99–B(6):759–65.
Acknowledgements
Not applicable.
Funding
This research was funded by the Research Grant (BRIC-2021 ID4) of the Italian Workers’ Compensation Authority (INAIL).
Author information
Authors and Affiliations
Contributions
AH and LA conceptualized the study, screened the literature, and wrote the first draft of the manuscript. LA performed the meta-analysis. KW, AP, FR, and GV contributed to the advanced draft of the study. RP and VD revised the final draft of the manuscript and supervised the whole study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huppert, A., Ambrosio, L., Nwosu, K. et al. Previous lumbar spine fusion increases the risk of dislocation following total hip arthroplasty in patients with hip-spine syndrome: a systematic review and meta-analysis. BMC Musculoskelet Disord 25, 732 (2024). https://doi.org/10.1186/s12891-024-07823-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-024-07823-1